







DOI: https://doi.org/10.37349/ebmx.2023.00001

Translating biomaterials research into clinical products is a multidisciplinary yet rewarding journey. It should primarily aim to improve patient treatment and clinical outcomes by addressing unmet clinical needs. Four examples of commercial development of biomaterial implants illustrate the diversity of paths, starting from academic research work (AlchiMedics, TISSIUM, Cousin Surgery) or corporate initiatives to develop new products (Medtronic-Sofradim Production). They have been selected from the Translational Research session of the 2022 Conference of the European Society for Biomaterials (ESB) in Bordeaux (France). Commitment, agility, and perseverance were among the key common skills to successfully meet challenges, especially the most unexpected ones. All dimensions of translation projects must be integrated from the start, including the regulatory strategy.
Translating biomaterials research into clinical products is a multidisciplinary yet rewarding journey. It should primarily aim to improve patient treatment and clinical outcomes by addressing unmet clinical needs. Four examples of commercial development of biomaterial implants illustrate the diversity of paths, starting from academic research work (AlchiMedics, TISSIUM, Cousin Surgery) or corporate initiatives to develop new products (Medtronic-Sofradim Production). They have been selected from the Translational Research session of the 2022 Conference of the European Society for Biomaterials (ESB) in Bordeaux (France). Commitment, agility, and perseverance were among the key common skills to successfully meet challenges, especially the most unexpected ones. All dimensions of translation projects must be integrated from the start, including the regulatory strategy.
DOI: https://doi.org/10.37349/ebmx.2024.00004

Aim:
Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, research on nerve guidence conduits is needed without drawbacks. The aim of the study was to develop hydrogel-based conduits containing interpenetrating network (IPN).
Methods:
Methacrylated gelatin (GelMA)-methacrylated hyaluronic acid (HaMA) IPN was filled the poly(2-hydroxyethylmethacrylate) (pHEMA) the outer conduit. Schwann cells (SCs) were used on the pHEMA and the distal end of the tube was injected with netrin-1 to support model SH-SY5Y cells.
Results:
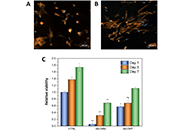
1H-nuclear magnetic resonance (1H-NMR) showed that methacrylation degrees were 94% ± 2% for GelMA and 60% ± 7% for HaMA. The fraction of HaMA increased the degradation rate; pure HaMA degraded in 3 weeks, while pure GelMA in more than 5 weeks. An increase in the fraction of 2-hydroxyethylmethacrylate (HEMA) from 20% to 56% decreased the porosity and the pore size, significantly. SH-SY5Y cells migrated along the conduit in the presence of netrin-1. NeuN expression was increased in 2 weeks indicating neuronal activity.
Conclusions:
SH-SY5Y cells produced neurites in the IPN. pHEMA conduit including GelMA-HaMA IPN is a good candidate for peripheral nerve regeneration applications. As future studies, the conduit will be tested in vivo for nerve regeneration.
Aim:
Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, research on nerve guidence conduits is needed without drawbacks. The aim of the study was to develop hydrogel-based conduits containing interpenetrating network (IPN).
Methods:
Methacrylated gelatin (GelMA)-methacrylated hyaluronic acid (HaMA) IPN was filled the poly(2-hydroxyethylmethacrylate) (pHEMA) the outer conduit. Schwann cells (SCs) were used on the pHEMA and the distal end of the tube was injected with netrin-1 to support model SH-SY5Y cells.
Results:
1H-nuclear magnetic resonance (1H-NMR) showed that methacrylation degrees were 94% ± 2% for GelMA and 60% ± 7% for HaMA. The fraction of HaMA increased the degradation rate; pure HaMA degraded in 3 weeks, while pure GelMA in more than 5 weeks. An increase in the fraction of 2-hydroxyethylmethacrylate (HEMA) from 20% to 56% decreased the porosity and the pore size, significantly. SH-SY5Y cells migrated along the conduit in the presence of netrin-1. NeuN expression was increased in 2 weeks indicating neuronal activity.
Conclusions:
SH-SY5Y cells produced neurites in the IPN. pHEMA conduit including GelMA-HaMA IPN is a good candidate for peripheral nerve regeneration applications. As future studies, the conduit will be tested in vivo for nerve regeneration.
DOI: https://doi.org/10.37349/ebmx.2024.00005
This article belongs to the special issue Nature-Based Biomaterials for Biomedical Applications

Aim:
The pleiotropic effect of fibroblast growth factor 2 (FGF2) on promoting myogenesis, angiogenesis, and innervation makes it an ideal growth factor for treating volumetric muscle loss (VML) injuries. While an initial delivery of FGF2 has demonstrated enhanced regenerative potential, the sustained delivery of FGF2 from scaffolds with robust structural properties as well as biophysical and biochemical signaling cues has yet to be explored for treating VML. The goal of this study is to develop an instructive fibrin microthread scaffold with intrinsic topographic alignment cues as well as regenerative signaling cues and a physiologically relevant, sustained release of FGF2 to direct myogenesis and ultimately enhance functional muscle regeneration.
Methods:
Heparin was passively adsorbed or carbodiimide-conjugated to microthreads, creating a biomimetic binding strategy, mimicking FGF2 sequestration in the extracellular matrix (ECM). It was also evaluated whether FGF2 incorporated into fibrin microthreads would yield sustained release. It was hypothesized that heparin-conjugated and co-incorporated (co-inc) fibrin microthreads would facilitate sustained release of FGF2 from the scaffold and enhance in vitro myoblast proliferation and outgrowth.
Results:
Toluidine blue staining and Fourier transform infrared spectroscopy confirmed that carbodiimide-conjugated heparin bound to fibrin microthreads in a dose-dependent manner. Release kinetics revealed that heparin-conjugated fibrin microthreads exhibited sustained release of FGF2 over a period of one week. An in vitro assay demonstrated that FGF2 released from microthreads remained bioactive, stimulating myoblast proliferation over four days. Finally, a cellular outgrowth assay suggests that FGF2 promotes increased outgrowth onto microthreads.
Conclusions:
It was anticipated that the combined effects of fibrin microthread structural properties, topographic alignment cues, and FGF2 release profiles will facilitate the fabrication of a biomimetic scaffold that enhances the regeneration of functional muscle tissue for the treatment of VML injuries.
Aim:
The pleiotropic effect of fibroblast growth factor 2 (FGF2) on promoting myogenesis, angiogenesis, and innervation makes it an ideal growth factor for treating volumetric muscle loss (VML) injuries. While an initial delivery of FGF2 has demonstrated enhanced regenerative potential, the sustained delivery of FGF2 from scaffolds with robust structural properties as well as biophysical and biochemical signaling cues has yet to be explored for treating VML. The goal of this study is to develop an instructive fibrin microthread scaffold with intrinsic topographic alignment cues as well as regenerative signaling cues and a physiologically relevant, sustained release of FGF2 to direct myogenesis and ultimately enhance functional muscle regeneration.
Methods:
Heparin was passively adsorbed or carbodiimide-conjugated to microthreads, creating a biomimetic binding strategy, mimicking FGF2 sequestration in the extracellular matrix (ECM). It was also evaluated whether FGF2 incorporated into fibrin microthreads would yield sustained release. It was hypothesized that heparin-conjugated and co-incorporated (co-inc) fibrin microthreads would facilitate sustained release of FGF2 from the scaffold and enhance in vitro myoblast proliferation and outgrowth.
Results:
Toluidine blue staining and Fourier transform infrared spectroscopy confirmed that carbodiimide-conjugated heparin bound to fibrin microthreads in a dose-dependent manner. Release kinetics revealed that heparin-conjugated fibrin microthreads exhibited sustained release of FGF2 over a period of one week. An in vitro assay demonstrated that FGF2 released from microthreads remained bioactive, stimulating myoblast proliferation over four days. Finally, a cellular outgrowth assay suggests that FGF2 promotes increased outgrowth onto microthreads.
Conclusions:
It was anticipated that the combined effects of fibrin microthread structural properties, topographic alignment cues, and FGF2 release profiles will facilitate the fabrication of a biomimetic scaffold that enhances the regeneration of functional muscle tissue for the treatment of VML injuries.
DOI: https://doi.org/10.37349/ebmx.2024.00006

Aim:
Since decades, decellularized extracellular matrix (dECM)-derived materials have received worldwide attention as promising biomaterials for tissue engineering and biomedical applications. Soluble dECM is a versatile raw material that can be easily engineered into the desired shapes and structures. However, there are still some limitations restricting its use, including low hydrophilicity and smooth surfaces, which negatively influence cell adhesion/spreading. The objective of the present study was to investigate surface modification by nitrogen/hydrogen (N2/H2) low-pressure cold plasma treatment as a potential technique to improve the biological response of bovine pericardium dECM films.
Methods:
Bovine pericardium dECM was enzymatically digested and lyophilized prior to the preparation of thin films via solvent-casting method. Changes in surface properties after plasma treatment were investigated using water contact angle (WCA) and X-ray photoelectron spectroscopy (XPS) measurements. Immunofluorescence staining and resazurin assay for human dermal fibroblasts (HDFs) cultured on the dECM films were used to assess the bioactivity of dECM films. Finally, the hemocompatibility of the films was investigated via clotting time and hemolysis assay.
Results:
WCA and XPS results revealed that oxygen (O)- and N-containing functional groups were incorporated onto the film surface and an increase in hydrophilicity was observed after plasma treatment. In vitro experiments showed that cell adhesion in plasma-treated dECM films is much faster if compared to the untreated controls. Moreover, the fibroblast proliferation increased after plasma surface modifications. Finally, the hemocompatibility analysis results indicated a delayed blood clotting and no hemolytic effects for all the tested samples.
Conclusions:
These findings confirmed the potential of dECM as raw material for biocompatible thin films fabrication. Additionally, plasma surface treatment emerged as an eco-friendly and cost-effective strategy to enhance in vitro cell attachment and proliferation on dECM films, expanding their applications in biomedicine.
Aim:
Since decades, decellularized extracellular matrix (dECM)-derived materials have received worldwide attention as promising biomaterials for tissue engineering and biomedical applications. Soluble dECM is a versatile raw material that can be easily engineered into the desired shapes and structures. However, there are still some limitations restricting its use, including low hydrophilicity and smooth surfaces, which negatively influence cell adhesion/spreading. The objective of the present study was to investigate surface modification by nitrogen/hydrogen (N2/H2) low-pressure cold plasma treatment as a potential technique to improve the biological response of bovine pericardium dECM films.
Methods:
Bovine pericardium dECM was enzymatically digested and lyophilized prior to the preparation of thin films via solvent-casting method. Changes in surface properties after plasma treatment were investigated using water contact angle (WCA) and X-ray photoelectron spectroscopy (XPS) measurements. Immunofluorescence staining and resazurin assay for human dermal fibroblasts (HDFs) cultured on the dECM films were used to assess the bioactivity of dECM films. Finally, the hemocompatibility of the films was investigated via clotting time and hemolysis assay.
Results:
WCA and XPS results revealed that oxygen (O)- and N-containing functional groups were incorporated onto the film surface and an increase in hydrophilicity was observed after plasma treatment. In vitro experiments showed that cell adhesion in plasma-treated dECM films is much faster if compared to the untreated controls. Moreover, the fibroblast proliferation increased after plasma surface modifications. Finally, the hemocompatibility analysis results indicated a delayed blood clotting and no hemolytic effects for all the tested samples.
Conclusions:
These findings confirmed the potential of dECM as raw material for biocompatible thin films fabrication. Additionally, plasma surface treatment emerged as an eco-friendly and cost-effective strategy to enhance in vitro cell attachment and proliferation on dECM films, expanding their applications in biomedicine.
DOI: https://doi.org/10.37349/ebmx.2024.00007

Neurodegenerative diseases (NDDs) gradually affect neurons impacting both their function and structure, and they afflict millions worldwide. Detecting these conditions before symptoms arise is crucial for better prognosis and duality of life, given that the disease processes often begin years earlier. Yet, reliable and affordable methods to diagnose NDDs in these stages are currently lacking. There’s a growing interest in using circulating extracellular vesicles (EVs), like small EVs (sEVs) also known as exosomes, as potential sources of markers for screening, diagnosing, and monitoring NDDs. This interest stems from evidence showing that these EVs can carry brain pathological proteins implicated in NDD pathology, and they can even traverse the blood-brain barrier. This review focuses on the creation of EVs, particularly sEVs with a size of less than 200 nanometers, methods for isolating sEVs, and recent advancements in biosensor development to detect NDD-related markers found in sEVs. Furthermore, it explores the potential of sEVs in diagnosing four major NDDs: Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and multiple system atrophy (MSA).
Neurodegenerative diseases (NDDs) gradually affect neurons impacting both their function and structure, and they afflict millions worldwide. Detecting these conditions before symptoms arise is crucial for better prognosis and duality of life, given that the disease processes often begin years earlier. Yet, reliable and affordable methods to diagnose NDDs in these stages are currently lacking. There’s a growing interest in using circulating extracellular vesicles (EVs), like small EVs (sEVs) also known as exosomes, as potential sources of markers for screening, diagnosing, and monitoring NDDs. This interest stems from evidence showing that these EVs can carry brain pathological proteins implicated in NDD pathology, and they can even traverse the blood-brain barrier. This review focuses on the creation of EVs, particularly sEVs with a size of less than 200 nanometers, methods for isolating sEVs, and recent advancements in biosensor development to detect NDD-related markers found in sEVs. Furthermore, it explores the potential of sEVs in diagnosing four major NDDs: Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and multiple system atrophy (MSA).
DOI: https://doi.org/10.37349/ebmx.2024.00008

Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
DOI: https://doi.org/10.37349/ebmx.2024.00009

Aim:
Polymethylmethacrylate bone cement is often used to reconstruct critical-sized defects generated by the surgical resection of spinal metastases. Residual tumor cells after a resection can drive recurrence and destabilization. Doxorubicin (DOX) is a common chemotherapeutic drug with unwanted side-effects when administered systemically. Mesoporous silica nanoparticles (NPs) are gaining attention for targeted drug delivery to bypass the negative side effects associated with systemic drug administration. An NP-functionalized cement was developed for the local release of DOX and its ability to suppress cancer cells was tested.
Methods:
DOX was loaded onto NPs which were then mixed into the cement. Static contact angles were measured. Drug release profiles were obtained over a period of 4 weeks. Cement constructs were incubated with two-dimensional (2D) cultures of human bone-marrow derived mesenchymal stem cells and human osteoblasts, as well as 2D and three-dimensional (3D) cultures of breast and prostate cancer cell lines. Cell metabolic activity and viability were evaluated. Cell migration and spheroid growth of cancer cell lines were assessed in collagen-coated spheroid cultures.
Results:
NPs were homogenously dispersed and did not alter the mechanical strength nor the wettability of the cement. A sustained DOX release profile was achieved with the addition of NPs to the bone cement. The release profile of DOX from NP cement may be modified by varying the amount of the drug loaded onto the NPs and the proportion of NPs in the cement. Cancer cells treated with the cement constructs showed a dose- and time-dependent inhibition, with minimal toxicity against healthy cells. Cancer cell migration and spheroid growth were impaired in 3D culture.
Conclusions:
NPs were shown to be essential for sustained DOX release from bone cement. DOX-loaded NP cement can inhibit cancer cells and impair their migration, with strong potential for in vivo translation studies.
Aim:
Polymethylmethacrylate bone cement is often used to reconstruct critical-sized defects generated by the surgical resection of spinal metastases. Residual tumor cells after a resection can drive recurrence and destabilization. Doxorubicin (DOX) is a common chemotherapeutic drug with unwanted side-effects when administered systemically. Mesoporous silica nanoparticles (NPs) are gaining attention for targeted drug delivery to bypass the negative side effects associated with systemic drug administration. An NP-functionalized cement was developed for the local release of DOX and its ability to suppress cancer cells was tested.
Methods:
DOX was loaded onto NPs which were then mixed into the cement. Static contact angles were measured. Drug release profiles were obtained over a period of 4 weeks. Cement constructs were incubated with two-dimensional (2D) cultures of human bone-marrow derived mesenchymal stem cells and human osteoblasts, as well as 2D and three-dimensional (3D) cultures of breast and prostate cancer cell lines. Cell metabolic activity and viability were evaluated. Cell migration and spheroid growth of cancer cell lines were assessed in collagen-coated spheroid cultures.
Results:
NPs were homogenously dispersed and did not alter the mechanical strength nor the wettability of the cement. A sustained DOX release profile was achieved with the addition of NPs to the bone cement. The release profile of DOX from NP cement may be modified by varying the amount of the drug loaded onto the NPs and the proportion of NPs in the cement. Cancer cells treated with the cement constructs showed a dose- and time-dependent inhibition, with minimal toxicity against healthy cells. Cancer cell migration and spheroid growth were impaired in 3D culture.
Conclusions:
NPs were shown to be essential for sustained DOX release from bone cement. DOX-loaded NP cement can inhibit cancer cells and impair their migration, with strong potential for in vivo translation studies.
DOI: https://doi.org/10.37349/ebmx.2024.00010

The use of autologous cartilage and bone grafts remains the gold standard in augmentation rhinoplasty performed to reconstruct of the nasal dorsum. Meanwhile, limited number of available sources, donor site morbidity, and unpredictable graft resorption represent significant disadvantages of autografting. The aim of this study is to test combination of autologous stromal vascular fraction (SVF) and commercially available bone substitutes (BSs) as new tissue-engineered grafting material (GM) for rhinoplasty. A series of consecutive cases includes four adult patients who underwent rhinoplasty to correct saddle nose deformity (SND) using the new graft technique. SVF was isolated from liposuction aspirate using standard methodology of enzymatic digestion. Two types of BSs were combined with SVF: Bio-Oss granules to create a moldable graft (M-graft), and block-shaped BoneMedik-S to create rigid grafts (R-grafts). The moderate SND was treated using an M-graft. In case of major or complex SND, the nasal dorsum was reconstructed with dorso-columellar L-shaped framework made of R-grafts. The results were evaluated over a period of 6 months to 3 years postoperatively using photogrammetry and FACE-Q appearance appraisal scales. Computerised tomography (CT) scanning of the reconstructed nose and histological analysis of grafted material were also carried out. No complications were observed. The photograms show the restoration of the correct contour of the nose. FACE-Q appraisal scale scores increased significantly, including satisfaction with nose appearance, psychological well-being, and social function. In CT evaluation, there was no substantial resorption or warping of the grafts. Histological findings show osteogenic remodeling of the grafted material. Thus, combining autologous SVF with BSs is a promising strategy for developing rhinoplasty GM.
The use of autologous cartilage and bone grafts remains the gold standard in augmentation rhinoplasty performed to reconstruct of the nasal dorsum. Meanwhile, limited number of available sources, donor site morbidity, and unpredictable graft resorption represent significant disadvantages of autografting. The aim of this study is to test combination of autologous stromal vascular fraction (SVF) and commercially available bone substitutes (BSs) as new tissue-engineered grafting material (GM) for rhinoplasty. A series of consecutive cases includes four adult patients who underwent rhinoplasty to correct saddle nose deformity (SND) using the new graft technique. SVF was isolated from liposuction aspirate using standard methodology of enzymatic digestion. Two types of BSs were combined with SVF: Bio-Oss granules to create a moldable graft (M-graft), and block-shaped BoneMedik-S to create rigid grafts (R-grafts). The moderate SND was treated using an M-graft. In case of major or complex SND, the nasal dorsum was reconstructed with dorso-columellar L-shaped framework made of R-grafts. The results were evaluated over a period of 6 months to 3 years postoperatively using photogrammetry and FACE-Q appearance appraisal scales. Computerised tomography (CT) scanning of the reconstructed nose and histological analysis of grafted material were also carried out. No complications were observed. The photograms show the restoration of the correct contour of the nose. FACE-Q appraisal scale scores increased significantly, including satisfaction with nose appearance, psychological well-being, and social function. In CT evaluation, there was no substantial resorption or warping of the grafts. Histological findings show osteogenic remodeling of the grafted material. Thus, combining autologous SVF with BSs is a promising strategy for developing rhinoplasty GM.
DOI: https://doi.org/10.37349/ebmx.2024.00011
This article belongs to the special issue Bioinspired Material for Regenerative Medicine

DOI: https://doi.org/10.37349/ebmx.2024.00012
This article belongs to the special issue Innovations in Biomaterials for Dentistry and Oral Surgery

Aim:
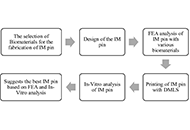
In this study, the finite elements analysis (FEA) was performed on an intramedullary (IM) pin to be used in the canine femur. The 03 different biomaterials [17-4-precipitated hardened (PH)-stainless steel (SS), nickel alloys (Ni)-625, titanium alloys (Ti)-6Al-4V] were selected for comparative FEA. In-vitro analysis was also performed in simulated body fluid (SBF) on selected biomaterials for possible application in the canine femur.
Methods:
FEA was performed on 03 different biomaterials (17-4-PH-SS, Ni-625, and Ti-6Al-4V) based on Von-Mises criteria (at an applied load of 1,500 N, cell type: tetrahedron, grit size: 0.15 mm, number of nodes: 213,989 and elements: 145,012). The distal end of the IM pin was fixed, and the load was applied to the proximal end. In-vitro analysis was performed (on a potentiostat setup) to establish the corrosion rate of various biomaterials (17-4-PH-SS, Ni-625, and Ti-6Al-4V).
Results:
The results of FEA show Ni-625 absorbed the maximum Von-Mises stress in the case of tensile and compression loading (104.12 MPa). In the case of torsion loading, the maximum Von-Mises stress was absorbed by 17-4-PH-SS (63.331 MPa). The maximum Von-Mises elastic strain (0.00093473) was observed for Ti-6Al-4V while tensile and compression and minimum deformation (0.013869 mm) in tensile loading.
Conclusions:
Based on this study, the maximum safety factor against failure (N) [ratio of 0.2% of yield strength (σy) to the Von-Mises stress (σv)] was observed as 10.75, 11.38, and 15.89, respectively, for tensile, compression, and torsional loading in the case of Ti-6Al-4V. Also, the better biocompatible material for the orthopaedic implant application based on the corrosion result is Ti-6Al-4V due to a lower corrosion rate (2.63211 × 10–10 mm/year) in comparison to 17-4-PH-SS and Ni-625. Overall, the Ti-6Al-4V is a better material than 17-4-PH-SS and Ni-625 for the intended application.
Aim:
In this study, the finite elements analysis (FEA) was performed on an intramedullary (IM) pin to be used in the canine femur. The 03 different biomaterials [17-4-precipitated hardened (PH)-stainless steel (SS), nickel alloys (Ni)-625, titanium alloys (Ti)-6Al-4V] were selected for comparative FEA. In-vitro analysis was also performed in simulated body fluid (SBF) on selected biomaterials for possible application in the canine femur.
Methods:
FEA was performed on 03 different biomaterials (17-4-PH-SS, Ni-625, and Ti-6Al-4V) based on Von-Mises criteria (at an applied load of 1,500 N, cell type: tetrahedron, grit size: 0.15 mm, number of nodes: 213,989 and elements: 145,012). The distal end of the IM pin was fixed, and the load was applied to the proximal end. In-vitro analysis was performed (on a potentiostat setup) to establish the corrosion rate of various biomaterials (17-4-PH-SS, Ni-625, and Ti-6Al-4V).
Results:
The results of FEA show Ni-625 absorbed the maximum Von-Mises stress in the case of tensile and compression loading (104.12 MPa). In the case of torsion loading, the maximum Von-Mises stress was absorbed by 17-4-PH-SS (63.331 MPa). The maximum Von-Mises elastic strain (0.00093473) was observed for Ti-6Al-4V while tensile and compression and minimum deformation (0.013869 mm) in tensile loading.
Conclusions:
Based on this study, the maximum safety factor against failure (N) [ratio of 0.2% of yield strength (σy) to the Von-Mises stress (σv)] was observed as 10.75, 11.38, and 15.89, respectively, for tensile, compression, and torsional loading in the case of Ti-6Al-4V. Also, the better biocompatible material for the orthopaedic implant application based on the corrosion result is Ti-6Al-4V due to a lower corrosion rate (2.63211 × 10–10 mm/year) in comparison to 17-4-PH-SS and Ni-625. Overall, the Ti-6Al-4V is a better material than 17-4-PH-SS and Ni-625 for the intended application.
DOI: https://doi.org/10.37349/ebmx.2024.00013
This article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and Implants

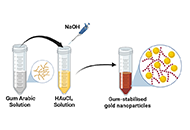
Aim:
To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing agent.
Methods:
Green synthesis of nanoparticles, characterization by absorption, infra-red and fluorescence spectroscopy.
Results:
The absorption spectrum (UV-Vis) showed an absorption peak ~522 nm corresponding to the surface plasmon resonance (SPR) absorption peak of AuNPs. Transmission electron microscopy (TEM) images revealed spherical-shaped nanoparticles with an average size of 15 nm. Fourier transform infrared (FTIR) analysis showed that the nanoparticles are coated with organic compounds that are present in GA. The fluorescence quenching properties of the AuNPs were assessed by monitoring their effects on fluorescence intensity of coumarin 153 (C153) dye. The fluorescence of the dye decreased with an increase in concentration of the nanoparticles. Upon addition of the protein bovine serum albumin (BSA) to the mixture the fluorescence increased (recovery) again.
Conclusions:
The fluorescence quenching and recovery (turn-on/off system) is a valuable method for protein detection in solution. By observing the effect of BSA on the quenched fluorescence, this nanoparticle system shows promise in biomedicine, drug delivery and environmental monitoring.
Aim:
To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing agent.
Methods:
Green synthesis of nanoparticles, characterization by absorption, infra-red and fluorescence spectroscopy.
Results:
The absorption spectrum (UV-Vis) showed an absorption peak ~522 nm corresponding to the surface plasmon resonance (SPR) absorption peak of AuNPs. Transmission electron microscopy (TEM) images revealed spherical-shaped nanoparticles with an average size of 15 nm. Fourier transform infrared (FTIR) analysis showed that the nanoparticles are coated with organic compounds that are present in GA. The fluorescence quenching properties of the AuNPs were assessed by monitoring their effects on fluorescence intensity of coumarin 153 (C153) dye. The fluorescence of the dye decreased with an increase in concentration of the nanoparticles. Upon addition of the protein bovine serum albumin (BSA) to the mixture the fluorescence increased (recovery) again.
Conclusions:
The fluorescence quenching and recovery (turn-on/off system) is a valuable method for protein detection in solution. By observing the effect of BSA on the quenched fluorescence, this nanoparticle system shows promise in biomedicine, drug delivery and environmental monitoring.
DOI: https://doi.org/10.37349/ebmx.2024.00014
This article belongs to the special issue Green Nanoparticles for Biomedical Applications

Aim:
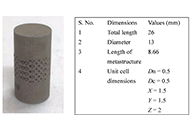
The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical properties, the 17-4 precipitate hardened (PH) stainless steel (SS) prototypes were successfully 3D printed by powder bed fusion (PBF) process with solid and octet metastructure to reduce stress shielding.
Methods:
The maxillary PM4 tooth of a male German shepherd dog was selected as the subject for the proposed study. As PM4 loading in canines is analogous to compressive loading conditions, finite element analysis (FEA) under compression was performed to compare simulated results of solid and octet meta-structure specimens. Solid and octet meta structure-based compression samples were prepared per ASTM E9 standard using SolidWorks software. The octet metastructure was designed with node and connector diameters of 0.5 mm each on 3DXpert software. Further FEA analysis of designed compression samples was performed using Ansys Workbench by selecting 17-4PH SS material at loading conditions of 800 N and 5,000 N.
Results:
The FEA results at the loading of 800 N show that maximum Von-Mises stress in the case of the solid and octet meta structure-based compression specimen was 10.029 MPa and 131.61 MPa, respectively. Further, the maximum Von-Mises strain for the solid and octet meta-structure-based specimens was 0.000049163 and 0.00067179, respectively. Similarly, deformation (in mm) for solid and octet truss lattice-based compression samples were 0.00075097 and 0.001451, respectively. The results observed at the loading condition of 5,000 N followed a pattern similar to that of 800 N loading conditions.
Conclusions:
Octet metastructure-based compression sample showed encouraging potential for withstanding maximum compression loading applicable to canine (800 N) while lowering the impacts of stress shielding. The safety factor against failure (N) was 4.33 and 62.31 for the octet meta-structure and solid compression samples, respectively.
Aim:
The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical properties, the 17-4 precipitate hardened (PH) stainless steel (SS) prototypes were successfully 3D printed by powder bed fusion (PBF) process with solid and octet metastructure to reduce stress shielding.
Methods:
The maxillary PM4 tooth of a male German shepherd dog was selected as the subject for the proposed study. As PM4 loading in canines is analogous to compressive loading conditions, finite element analysis (FEA) under compression was performed to compare simulated results of solid and octet meta-structure specimens. Solid and octet meta structure-based compression samples were prepared per ASTM E9 standard using SolidWorks software. The octet metastructure was designed with node and connector diameters of 0.5 mm each on 3DXpert software. Further FEA analysis of designed compression samples was performed using Ansys Workbench by selecting 17-4PH SS material at loading conditions of 800 N and 5,000 N.
Results:
The FEA results at the loading of 800 N show that maximum Von-Mises stress in the case of the solid and octet meta structure-based compression specimen was 10.029 MPa and 131.61 MPa, respectively. Further, the maximum Von-Mises strain for the solid and octet meta-structure-based specimens was 0.000049163 and 0.00067179, respectively. Similarly, deformation (in mm) for solid and octet truss lattice-based compression samples were 0.00075097 and 0.001451, respectively. The results observed at the loading condition of 5,000 N followed a pattern similar to that of 800 N loading conditions.
Conclusions:
Octet metastructure-based compression sample showed encouraging potential for withstanding maximum compression loading applicable to canine (800 N) while lowering the impacts of stress shielding. The safety factor against failure (N) was 4.33 and 62.31 for the octet meta-structure and solid compression samples, respectively.
DOI: https://doi.org/10.37349/ebmx.2024.00015
This article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and Implants

Aim:
This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart valve leaflets in structure, composition, and mechanical properties, among which, the bending anisotropic behaviour in both the with curvature (WC) and the against curvature (AC) directions, is the most desired. The use of atelocollagen is of significant importance in highlighting the non-antigenic potential of the design.
Methods:
Porous scaffolds were freeze-dried, then crosslinked using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The fibrillogenesis occurrence and the scaffold microstructure were imaged using scanning electron microscopy (SEM). Fourier transform infrared spectroscopy (FITR) investigated the effect of fabrication and crosslinking on the backbone structure. Dynamic mechanical analysis (DMA) characterised the compressive and bending properties of the scaffolds in hydrated and non-hydrated states. Three-point bending and a “self-deflection” test were performed on tri-layer scaffolds in both WC and AC directions.
Results:
Atelocollagen-based scaffolds were successfully produced, rendering this study the first to report a tri-layer structure using atelocollagen, HA, and elastin fibrillar gel. The scaffolds’ porosity was tailored to accommodate potential future biological studies and the transition between layers appeared seamless. FITR unveiled effective crosslinking and the backbone structure preservation. The scaffolds exhibited lightly crosslinked polymer resembling mechanical responses when non-hydrated, and the desired J-curve stress-strain response was observed when hydrated. The tri-layer scaffolds showed anisotropic bending behaviour with a bending modulus of 5.41 ± 1.14 kPa (WC) and 7.98 ± 2.22 kPa (AC).
Conclusions:
The tri-layer scaffolds fabricated resemble the native aortic valve leaflets in structure and composition, and successfully introduced bending anisotropy in physiological conditions. Together with the suitable microstructure and promising mechanical properties, the design is reckoned to be a potential tissue engineering heart valve candidate.
Aim:
This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart valve leaflets in structure, composition, and mechanical properties, among which, the bending anisotropic behaviour in both the with curvature (WC) and the against curvature (AC) directions, is the most desired. The use of atelocollagen is of significant importance in highlighting the non-antigenic potential of the design.
Methods:
Porous scaffolds were freeze-dried, then crosslinked using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The fibrillogenesis occurrence and the scaffold microstructure were imaged using scanning electron microscopy (SEM). Fourier transform infrared spectroscopy (FITR) investigated the effect of fabrication and crosslinking on the backbone structure. Dynamic mechanical analysis (DMA) characterised the compressive and bending properties of the scaffolds in hydrated and non-hydrated states. Three-point bending and a “self-deflection” test were performed on tri-layer scaffolds in both WC and AC directions.
Results:
Atelocollagen-based scaffolds were successfully produced, rendering this study the first to report a tri-layer structure using atelocollagen, HA, and elastin fibrillar gel. The scaffolds’ porosity was tailored to accommodate potential future biological studies and the transition between layers appeared seamless. FITR unveiled effective crosslinking and the backbone structure preservation. The scaffolds exhibited lightly crosslinked polymer resembling mechanical responses when non-hydrated, and the desired J-curve stress-strain response was observed when hydrated. The tri-layer scaffolds showed anisotropic bending behaviour with a bending modulus of 5.41 ± 1.14 kPa (WC) and 7.98 ± 2.22 kPa (AC).
Conclusions:
The tri-layer scaffolds fabricated resemble the native aortic valve leaflets in structure and composition, and successfully introduced bending anisotropy in physiological conditions. Together with the suitable microstructure and promising mechanical properties, the design is reckoned to be a potential tissue engineering heart valve candidate.
DOI: https://doi.org/10.37349/ebmx.2024.00016
This article belongs to the special issue Bioinspired Material for Regenerative Medicine

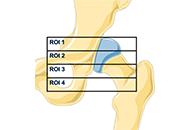
The increasing number of prosthetic hip replacement surgeries and their growing indication have led to a growing interest in understanding the factors that influence their long-term success. Total hip arthroplasty (THA) failure is mainly due to aseptic loosening. More rarely septic mobilization may occur. In the first case, many variables influence the bone-implant relationship and periprosthetic bone remodeling. Stress-shielding is the most evident but not fully explained manifestation of the bone implant interaction. Recently, three-dimensional (3D) printed titanium orthopedic implants have offered new perspectives in the field of hip prosthetics, enabling the customization and production of acetabular cups with enhanced biocompatibility. This review aims to evaluate the efficacy and reliability of 3D printed acetabular cups from the perspective of aseptic failure particularly related to the stress-shielding. The most recent clinical and preclinical studies will be reviewed, exploring the benefits and challenges associated with the use of these emerging technologies. Key factors, such as biocompatibility, mechanical stability, osseointegration, and wear resistance.
The increasing number of prosthetic hip replacement surgeries and their growing indication have led to a growing interest in understanding the factors that influence their long-term success. Total hip arthroplasty (THA) failure is mainly due to aseptic loosening. More rarely septic mobilization may occur. In the first case, many variables influence the bone-implant relationship and periprosthetic bone remodeling. Stress-shielding is the most evident but not fully explained manifestation of the bone implant interaction. Recently, three-dimensional (3D) printed titanium orthopedic implants have offered new perspectives in the field of hip prosthetics, enabling the customization and production of acetabular cups with enhanced biocompatibility. This review aims to evaluate the efficacy and reliability of 3D printed acetabular cups from the perspective of aseptic failure particularly related to the stress-shielding. The most recent clinical and preclinical studies will be reviewed, exploring the benefits and challenges associated with the use of these emerging technologies. Key factors, such as biocompatibility, mechanical stability, osseointegration, and wear resistance.
DOI: https://doi.org/10.37349/ebmx.2024.00017
This article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and Implants

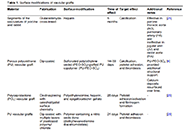
Improving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification of the biomaterials from which the vascular grafts are constructed has been used to reduce the risk of complications such as thrombosis and infection. Herein with a focus on vascular tissue engineering, we provided an overview of (a) fundamental hemodynamic considerations for blood-contacting biomaterials, (b) surface modification strategies to attenuate nonspecific adhesion of proteins, improve hemocompatibility, and induce the formation of a confluent endothelial lining, and (c) the guidelines for the clinical development of surface modified biomaterials.
Improving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification of the biomaterials from which the vascular grafts are constructed has been used to reduce the risk of complications such as thrombosis and infection. Herein with a focus on vascular tissue engineering, we provided an overview of (a) fundamental hemodynamic considerations for blood-contacting biomaterials, (b) surface modification strategies to attenuate nonspecific adhesion of proteins, improve hemocompatibility, and induce the formation of a confluent endothelial lining, and (c) the guidelines for the clinical development of surface modified biomaterials.
DOI: https://doi.org/10.37349/ebmx.2024.00018

Aim:
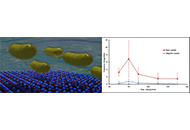
This study aimed to examine the amount of surface non-specific adsorption, or fouling, observed by Pseudomonas aeruginosa (P. aeruginosa) on a quartz crystal based acoustic wave biosensor under different flow conditions with and without an anti-fouling layer.
Methods:
An electromagnetic piezoelectric acoustic sensor (EMPAS) based on electrode free quartz crystals was used to perform the analysis. Phosphate buffered saline (PBS) was flowed over the crystal surface at various flow rates from 50 μL/min to 200 μL/min, with measurements being taken at the 43rd harmonic (~864 MHz). The crystal was either unmodified, or modified with a monoethylene glycol [2-(3-silylpropyloxy)-hydroxy-ethyl (MEG-OH)] anti-fouling layer. Overnight culture of P. aeruginosa PAO1 (PAO1) in lysogeny broth (LB) was injected into the system, and flow maintained for 30 min.
Results:
The frequency change of the EMPAS crystal after injection of bacteria into the system was found to change based on the flow rate of buffer, suggesting the flow rate has a strong effect on the level of non-specific adsorption. The MEG-OH layer drastically reduced the level of fouling observed under all flow conditions, as well as reduced the amount of variation between experiments. Flow rates of 150 μL/min or higher were found to best reduce the level of fouling observed as well as experimental variation.
Conclusions:
The MEG-OH anti-fouling layer is important for accurate and reproducible biosensing measurements due to the reduced fouling and variation during experiments. Additionally, a flow rate of 150 μL/min may prove better for measurement compared to the current standard of 50 μL/min for this type of instrument.
Aim:
This study aimed to examine the amount of surface non-specific adsorption, or fouling, observed by Pseudomonas aeruginosa (P. aeruginosa) on a quartz crystal based acoustic wave biosensor under different flow conditions with and without an anti-fouling layer.
Methods:
An electromagnetic piezoelectric acoustic sensor (EMPAS) based on electrode free quartz crystals was used to perform the analysis. Phosphate buffered saline (PBS) was flowed over the crystal surface at various flow rates from 50 μL/min to 200 μL/min, with measurements being taken at the 43rd harmonic (~864 MHz). The crystal was either unmodified, or modified with a monoethylene glycol [2-(3-silylpropyloxy)-hydroxy-ethyl (MEG-OH)] anti-fouling layer. Overnight culture of P. aeruginosa PAO1 (PAO1) in lysogeny broth (LB) was injected into the system, and flow maintained for 30 min.
Results:
The frequency change of the EMPAS crystal after injection of bacteria into the system was found to change based on the flow rate of buffer, suggesting the flow rate has a strong effect on the level of non-specific adsorption. The MEG-OH layer drastically reduced the level of fouling observed under all flow conditions, as well as reduced the amount of variation between experiments. Flow rates of 150 μL/min or higher were found to best reduce the level of fouling observed as well as experimental variation.
Conclusions:
The MEG-OH anti-fouling layer is important for accurate and reproducible biosensing measurements due to the reduced fouling and variation during experiments. Additionally, a flow rate of 150 μL/min may prove better for measurement compared to the current standard of 50 μL/min for this type of instrument.
DOI: https://doi.org/10.37349/ebmx.2023.00002

Aim:
This study aims to discover an alternative precursor with abundant source and low cost for multicolor graphene quantum dots (GQDs) preparation and application.
Methods:
In the current study, anthracite-derived multicolor GQDs were prepared at different reaction temperatures (100°–150°C), referring to the GQDs 100, GQDs 120, GQDs 130, and GQDs 150.
Results:
The GQDs 100, GQDs 120, GQDs 130, and GQDs 150 solutions were found to be orange-red, yellow-green, green, and blue under 365 nm excitation UV (ultraviolet) lamp, respectively. The X-ray photoelectron spectroscopy (XPS) data suggests high temperature intensifies oxidation of the amorphous sp3 carbon, resulting in GQDs with higher crystalline structure (Csp2). Compared with the GQDs 100 and GQDs 120, the GQDs 130 and GQDs 150 showed much better biocompatibility, which may attribute to their higher Csp2 composition and smaller size.
Conclusions:
The results suggest that GQDs 130 and GQDs 150 are ideal candidates for nanomedicine applications, e.g., drug/gene delivery and bio-imaging, etc.
Aim:
This study aims to discover an alternative precursor with abundant source and low cost for multicolor graphene quantum dots (GQDs) preparation and application.
Methods:
In the current study, anthracite-derived multicolor GQDs were prepared at different reaction temperatures (100°–150°C), referring to the GQDs 100, GQDs 120, GQDs 130, and GQDs 150.
Results:
The GQDs 100, GQDs 120, GQDs 130, and GQDs 150 solutions were found to be orange-red, yellow-green, green, and blue under 365 nm excitation UV (ultraviolet) lamp, respectively. The X-ray photoelectron spectroscopy (XPS) data suggests high temperature intensifies oxidation of the amorphous sp3 carbon, resulting in GQDs with higher crystalline structure (Csp2). Compared with the GQDs 100 and GQDs 120, the GQDs 130 and GQDs 150 showed much better biocompatibility, which may attribute to their higher Csp2 composition and smaller size.
Conclusions:
The results suggest that GQDs 130 and GQDs 150 are ideal candidates for nanomedicine applications, e.g., drug/gene delivery and bio-imaging, etc.
DOI: https://doi.org/10.37349/ebmx.2023.00003

Aim:
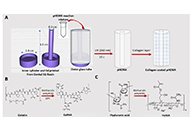
In this study, the physicochemical properties of stearyl glycyrrhetinate/β-cyclodextrin (SG/βCD) and SG/γCD complexes were characterized, and the complexes were prepared using the co-milling method. The molecular interactions within the SG/CD complexes were also investigated using nuclear magnetic resonance (NMR) measurements to determine the mode of interaction.
Methods:
Here, we evaluated the physicochemical properties of SG complexes with CDs prepared by ground mixtures (GM SG/βCD or γCD = 1/1, 1/2).
Results:
Powder X-ray diffraction (PXRD) showed that the characteristic SG and CD peaks disappeared after co-grinding with GM (SG/CD = molar ratio of 1/2), indicating a halo pattern. Differential scanning calorimetry (DSC) measurements showed that after co-grinding, the endothermic peak due to SG melting, as well as the dehydration peak and the endothermic peak due to SG melting, disappeared. Near-infrared (NIR) spectroscopy results showed that the peaks of SG-derived –CH moieties and CD-derived –OH and –CH moieties broadened in GM, suggesting their involvement in complex formation through SG/CDs intermolecular interactions. In GM (SG/CDs), NMR measurements showed broadened H-A and H-F peaks of the steroid skeleton derived from SG. In GM (SG/βCD = 1/2), the doublet peak, especially OH-3 at the wide edge of CD, shifted to a singlet peak. In GM (SG/γCD = 1/2), the H-3 peak, which is the wide edge of γCD, and the H-6 peak, which is the narrow edge, shifted to broad peaks, suggesting that γCD is deeply encapsulated in the steroidal structure.
Conclusions:
These findings suggest that complex formation occurred in SG/CDs and that inclusion behavior is different between GM (SG/βCD = 1/2) and GM (SG/γCD = 1/2).
Aim:
In this study, the physicochemical properties of stearyl glycyrrhetinate/β-cyclodextrin (SG/βCD) and SG/γCD complexes were characterized, and the complexes were prepared using the co-milling method. The molecular interactions within the SG/CD complexes were also investigated using nuclear magnetic resonance (NMR) measurements to determine the mode of interaction.
Methods:
Here, we evaluated the physicochemical properties of SG complexes with CDs prepared by ground mixtures (GM SG/βCD or γCD = 1/1, 1/2).
Results:
Powder X-ray diffraction (PXRD) showed that the characteristic SG and CD peaks disappeared after co-grinding with GM (SG/CD = molar ratio of 1/2), indicating a halo pattern. Differential scanning calorimetry (DSC) measurements showed that after co-grinding, the endothermic peak due to SG melting, as well as the dehydration peak and the endothermic peak due to SG melting, disappeared. Near-infrared (NIR) spectroscopy results showed that the peaks of SG-derived –CH moieties and CD-derived –OH and –CH moieties broadened in GM, suggesting their involvement in complex formation through SG/CDs intermolecular interactions. In GM (SG/CDs), NMR measurements showed broadened H-A and H-F peaks of the steroid skeleton derived from SG. In GM (SG/βCD = 1/2), the doublet peak, especially OH-3 at the wide edge of CD, shifted to a singlet peak. In GM (SG/γCD = 1/2), the H-3 peak, which is the wide edge of γCD, and the H-6 peak, which is the narrow edge, shifted to broad peaks, suggesting that γCD is deeply encapsulated in the steroidal structure.
Conclusions:
These findings suggest that complex formation occurred in SG/CDs and that inclusion behavior is different between GM (SG/βCD = 1/2) and GM (SG/γCD = 1/2).
DOI: https://doi.org/10.37349/ebmx.2025.101330
This article belongs to the special issue Nature-Based Biomaterials for Biomedical Applications

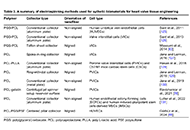
A potential solution for prosthetic heart valves is tissue-engineered heart valves. Tissue-engineered heart valves (TEHVs) are designed to replicate the complex properties found in natural tissues, such as stiffness, anisotropy, and composition and organization of cells and extracellular matrix (ECM). Electrospinning is regarded as a highly versatile and innovative approach for fabricating numerous fibrous designs. In this review, we discuss recent developments in electrospun heart valve scaffolds, including scaffold materials, cell types, and electrospinning setups used to prepare aligned nanofibers. Despite the fact that natural biomaterials provided excellent biocompatibility, nanofibers from synthetic materials provided the required mechanical compatibility. Accordingly, most studies highlighted the benefits of designing composite heart valves using biological and synthetic polymers. Various strategies, such as the application of motorized mandrel and micropatterned collector in electrospinning were effective in controlling nanofiber alignment. Studies also showed that aligned nanofiber’s mechanical strength and anisotropic structure promote cell proliferation, and differentiation, and promote attachment. Numerous studies have reported that multiple cell sources are suitable for producing heart valves. Successful results were obtained with human umbilical vein endothelial cells (HUVECs), since they provide a convenient cell source for cellularization of valve leaflets. A higher conductivity of scaffolds was achieved by using biomaterials that conduct electricity, such as polyaniline, polypyrrole, and carbon nanotubes, which resulted in better differentiation of precursor cells to cardiomyocytes and higher cell beating rates. In light of these attributes, nanofibrous scaffolds produced through electrospinning are expected to offer numerous advantages for tissue engineering and medical applications in the near future. However, multiple challenges were identified as cell infiltration and 2D nature of nanofiber mats necessitate further engineering approaches in electrospinning procedure leaflet production.
A potential solution for prosthetic heart valves is tissue-engineered heart valves. Tissue-engineered heart valves (TEHVs) are designed to replicate the complex properties found in natural tissues, such as stiffness, anisotropy, and composition and organization of cells and extracellular matrix (ECM). Electrospinning is regarded as a highly versatile and innovative approach for fabricating numerous fibrous designs. In this review, we discuss recent developments in electrospun heart valve scaffolds, including scaffold materials, cell types, and electrospinning setups used to prepare aligned nanofibers. Despite the fact that natural biomaterials provided excellent biocompatibility, nanofibers from synthetic materials provided the required mechanical compatibility. Accordingly, most studies highlighted the benefits of designing composite heart valves using biological and synthetic polymers. Various strategies, such as the application of motorized mandrel and micropatterned collector in electrospinning were effective in controlling nanofiber alignment. Studies also showed that aligned nanofiber’s mechanical strength and anisotropic structure promote cell proliferation, and differentiation, and promote attachment. Numerous studies have reported that multiple cell sources are suitable for producing heart valves. Successful results were obtained with human umbilical vein endothelial cells (HUVECs), since they provide a convenient cell source for cellularization of valve leaflets. A higher conductivity of scaffolds was achieved by using biomaterials that conduct electricity, such as polyaniline, polypyrrole, and carbon nanotubes, which resulted in better differentiation of precursor cells to cardiomyocytes and higher cell beating rates. In light of these attributes, nanofibrous scaffolds produced through electrospinning are expected to offer numerous advantages for tissue engineering and medical applications in the near future. However, multiple challenges were identified as cell infiltration and 2D nature of nanofiber mats necessitate further engineering approaches in electrospinning procedure leaflet production.
DOI: https://doi.org/10.37349/ebmx.2025.101331
This article belongs to the special issue Trends in Biomaterials Research for Cardiovascular Applications

