
Open Access
Editorial
Message of welcome from the editor-in-chief
Stefan R. Bornstein
Published: April 01, 2024 Explor Endocr Metab Dis. 2024;1:1–3
Open Access
Original Article
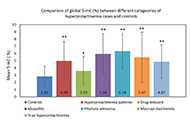
Epigenetics in etiopathology of hyperprolactinemia
Amanpreet Kaur Kalsi ... Jai Bhagwan Sharma
Published: May 22, 2024 Explor Endocr Metab Dis. 2024;1:39–55
This article belongs to the special issue The HPA Axis in Health and Disease
Open Access
Perspective
Diabetes and cancer: two epidemic diseases requiring an opposite therapeutic approach to target cells
Katrin Sak
Published: May 23, 2024 Explor Endocr Metab Dis. 2024;1:56–61

Open Access
Original Article
Four hepatic steatosis indices in predicting quantitative computed tomography-based metabolic dysfunction-associated fatty liver disease
Bingwu Xu ... Yong Zhang
Published: May 23, 2024 Explor Endocr Metab Dis. 2024;1:62–76
This article belongs to the special issue Regulators of Glucose Homeostasis, Lipid Metabolism and Energy Balance
Open Access
Case Report
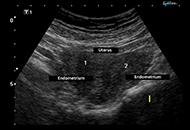
Hypoparathyroidism, deafness and renal dysplasia syndrome with bilateral cataract and bicornuate uterus caused by a de novo GATA3 mutation
Rajesh Chetiwal ... Priyank Rastogi
Published: May 27, 2024 Explor Endocr Metab Dis. 2024;1:77–82
Open Access
Review
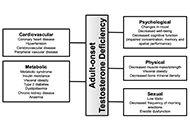
Adult-onset testosterone deficiency: the usefulness of hormone replacement in reducing mortality in men with this common age-related condition
Amar Mann ... Sudarshan Ramachandran
Published: June 28, 2024 Explor Endocr Metab Dis. 2024;1:83–100
This article belongs to the special issue The Fountain of Youth: Decoding the Hormonal Regulation of Aging
Open Access
Review
Genome editing in the adrenal gland: a novel strategy for treating congenital adrenal hyperplasia
Eva B. van Dijk ... Lara E. Graves
Published: July 09, 2024 Explor Endocr Metab Dis. 2024;1:101–121
This article belongs to the special issue The HPA Axis in Health and Disease
Open Access
Review
Hypothetical involvement of stress hormones-induced reprograming of adult stem/progenitor cells in tumorigenesis
Waldemar Kanczkowski ... George P. Chrousos
Published: July 15, 2024 Explor Endocr Metab Dis. 2024;1:122–157
This article belongs to the special issue The HPA Axis in Health and Disease

Open Access
Commentary
The 2024 American Diabetes Association guidelines on Standards of Medical Care in Diabetes: key takeaways for laboratory
Dipti Tiwari, Tar Choon Aw
Published: July 23, 2024 Explor Endocr Metab Dis. 2024;1:158–166

Open Access
Original Article
Glycemic trends, app engagement and achievement of gestational diabetes guideline targets using a diabetes app and Bluetooth® connected blood glucose meters
Mike Grady ... Elizabeth Holt
Published: July 24, 2024 Explor Endocr Metab Dis. 2024;1:167–176
This article belongs to the special issue The Impact of Digitalization To Improve Nutrition and Self-Management in Patients With Diabetes

Open Access
Original Article
Testosterone undecanoate is associated with improved ageing male symptoms score in men with type 2 diabetes and adult-onset testosterone deficiency: re-analyzed results from a randomised controlled trial
Pravinath Ramachandran ... Geoffrey Hackett
Published: August 13, 2024 Explor Endocr Metab Dis. 2024;1:177–190
This article belongs to the special issue The Fountain of Youth: Decoding the Hormonal Regulation of Aging
Open Access
Review
Endogenous glucocorticoids during skeletal ageing
Eugenie Macfarlane ... Markus Joachim Seibel
Published: August 16, 2024 Explor Endocr Metab Dis. 2024;1:191–212
This article belongs to the special issue The Fountain of Youth: Decoding the Hormonal Regulation of Aging

Open Access
Review
Application of chimeric antigen receptor-natural killer cells for the treatment of type 1 diabetes
Charlotte Steenblock ... Stefan R. Bornstein
Published: April 01, 2024 Explor Endocr Metab Dis. 2024;1:4–11
Open Access
Commentary
Unique original endocrine findings: the endoplasmic reticulum-mitochondrial unit in steroid producing cells
Stefan R. Bornstein ... Waldemar Kanczkowski
Published: April 01, 2024 Explor Endocr Metab Dis. 2024;1:12–15
This article belongs to the special issue The HPA Axis in Health and Disease
Open Access
Review
Recent advances in artificial intelligence-assisted endocrinology and diabetes
Ioannis T. Oikonomakos ... Stefan R. Bornstein
Published: April 01, 2024 Explor Endocr Metab Dis. 2024;1:16–26
Open Access
Original Article
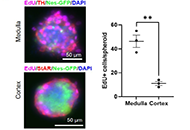
Development of adrenal 3-dimensional spheroid cultures: potential for the treatment of adrenal insufficiency and neurodegenerative diseases
Charlotte Steenblock ... Nicole Bechmann
Published: April 01, 2024 Explor Endocr Metab Dis. 2024;1:27–38
Open Access
Review
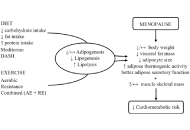
Healthy adipose tissue after menopause: contribution of balanced diet and physical exercise
Bruno Vecchiatto ... Fabiana S. Evangelista
Published: March 13, 2025 Explor Endocr Metab Dis. 2025;2:101424
This article belongs to the special issue Metabolic Syndrome in Menopause
Open Access
Review
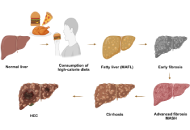
Significance of FXR agonists in MASLD treatment: a deep dive into lipid alteration by analytical techniques
Pirangi Srikanth ... Sukhendu Nandi
Published: March 25, 2025 Explor Endocr Metab Dis. 2025;2:101425
This article belongs to the special issue Regulators of Glucose Homeostasis, Lipid Metabolism and Energy Balance

Open Access
Review
Glucocorticoid receptor alpha: origins and functions of the master regulator of homeostatic corrections in health and critical illness
Gianfranco Umberto Meduri
Published: March 28, 2025 Explor Endocr Metab Dis. 2025;2:101426
Open Access
Review
Circulating endocannabinoids and brain anatomy: unraveling the weight loss connection through lifestyle and surgery approaches
Gabrielle St-Arnaud ... Vincenzo Di Marzo
Published: April 07, 2025 Explor Endocr Metab Dis. 2025;2:101427
This article belongs to the special issue Regulators of Glucose Homeostasis, Lipid Metabolism and Energy Balance

