Open Access
Original Article
Tumor burden as possible biomarker of outcome in advanced NSCLC patients treated with immunotherapy: a single center, retrospective, real-world analysis
Edoardo Lenci ... Rossana Berardi
Published: June 28, 2021 Explor Target Antitumor Ther. 2021;2:227–239
This article belongs to the special issue Immunotherapy in Cancer Patients
Open Access
Case Report
Lynch syndrome-associated lung cancer: pitfalls of an immunotherapy-based treatment strategy in an unusual tumor type
Elena Maccaroni ... Rossana Berardi
Published: June 28, 2021 Explor Target Antitumor Ther. 2021;2:240–248
Open Access
Review
Overview of Ca2+ signaling in lung cancer progression and metastatic lung cancer with bone metastasis
Manh Tien Tran
Published: June 28, 2021 Explor Target Antitumor Ther. 2021;2:249–265
This article belongs to the special issue Calcium Signaling Apparatus in Cancers

Open Access
Review
Advances in the study of cancer metastasis and calcium signaling as potential therapeutic targets
Chaochu Cui ... Xianwei Wang
Published: June 28, 2021 Explor Target Antitumor Ther. 2021;2:266–291
This article belongs to the special issue Calcium Signaling Apparatus in Cancers
Open Access
Review
Targeting cytoskeletal phosphorylation in cancer
Clara Llorente-González ... Miguel Vicente-Manzanares
Published: June 28, 2021 Explor Target Antitumor Ther. 2021;2:292–308

Open Access
Original Article
The effect of iron on the expression levels of calcium related gene in cisplatin resistant epithelial ovarian cancer cells
Bahire Kucukkaya ... Leman Yalcintepe
Published: August 30, 2021 Explor Target Antitumor Ther. 2021;2:309–322
Open Access
Review
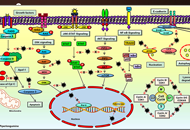
The promising potential of piperlongumine as an emerging therapeutics for cancer
Dey Parama ... Ajaikumar B. Kunnumakkara
Published: August 30, 2021 Explor Target Antitumor Ther. 2021;2:323–354
Open Access
Review
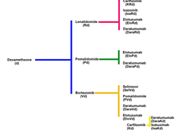
The evolving role and utility of off-label drug use in multiple myeloma
James H Stoeckle ... Gareth J Morgan
Published: August 30, 2021 Explor Target Antitumor Ther. 2021;2:355–373
This article belongs to the special issue Off-Label Drugs and -Omics Data in Cancer Treatment
Open Access
Review
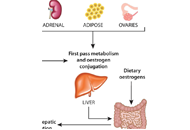
Does the gut microbiome environment influence response to systemic breast cancer treatment?
Eilidh Bruce ... Beatrix Elsberger
Published: August 30, 2021 Explor Target Antitumor Ther. 2021;2:374–384
Open Access
Review
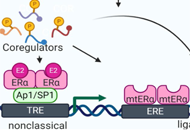
Role of estrogen receptor coregulators in endocrine resistant breast cancer
Kristin A. Altwegg, Ratna K. Vadlamudi
Published: August 30, 2021 Explor Target Antitumor Ther. 2021;2:385–400
This article belongs to the special issue Endocrine Resistant Breast Cancer
Open Access
Review
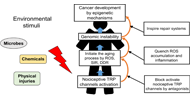
A novel strategy for treating cancer: understanding the role of Ca2+ signaling from nociceptive TRP channels in regulating cancer progression
Wen-Li Hsu ... Etsuro Ito
Published: October 31, 2021 Explor Target Antitumor Ther. 2021;2:401–415
This article belongs to the special issue Calcium Signaling Apparatus in Cancers
Open Access
Review
Current options and future directions of systemic therapy for advanced biliary tract cancer
Maria Giuseppina Prete ... Lorenza Rimassa
Published: October 31, 2021 Explor Target Antitumor Ther. 2021;2:416–433
This article belongs to the special issue Precision Medicine for Cholangiocarcinoma

Open Access
Review
Targeting protein kinase CK2 in the treatment of cholangiocarcinoma
Padma-Sheela Jayaraman, Kevin Gaston
Published: October 31, 2021 Explor Target Antitumor Ther. 2021;2:434–447
This article belongs to the special issue Precision Medicine for Cholangiocarcinoma
Open Access
Review
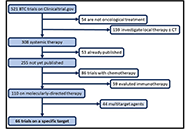
A review of molecularly targeted therapy in biliary tract carcinoma: what is the next step?
Giacomo Aimar ... Massimo Di Maio
Published: October 31, 2021 Explor Target Antitumor Ther. 2021;2:448–464
This article belongs to the special issue Precision Medicine for Cholangiocarcinoma

Open Access
Review
Targeted therapy of multiple myeloma
Shan Zhou, Renxi Wang
Published: October 31, 2021 Explor Target Antitumor Ther. 2021;2:465–480
Open Access
Original Article
Performing oncological procedures during COVID-19 outbreak: a picture from an Italian cancer center
Maristella Bungaro ... Silvia Novello
Published: October 31, 2021 Explor Target Antitumor Ther. 2021;2:481–489
This article belongs to the special issue COVID-19 and Cancer
Open Access
Review
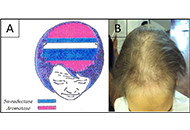
Clinical aspect, pathogenesis and therapy options of alopecia induced by hormonal therapy for breast cancer
Alfredo Rossi ... Marta Carlesimo
Published: October 31, 2021 Explor Target Antitumor Ther. 2021;2:490–495
This article belongs to the special issue Endocrine Resistant Breast Cancer
Open Access
Review
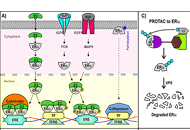
Proteolysis-targeting chimeras and their implications in breast cancer
Angeles C. Tecalco-Cruz ... Alberto Rojas-Ochoa
Published: December 31, 2021 Explor Target Antitumor Ther. 2021;2:496–510
This article belongs to the special issue Proteolysis Targeting Chimera (PROTAC)
Open Access
Review
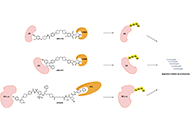
The clinical advances of proteolysis targeting chimeras in oncology
Hao Xie ... Jason B. Fleming
Published: December 31, 2021 Explor Target Antitumor Ther. 2021;2:511–521
This article belongs to the special issue Proteolysis Targeting Chimera (PROTAC)
Open Access
Review
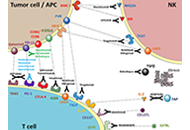
Immunotherapy in head and neck squamous cell carcinoma and rare head and neck malignancies
Stefano Cavalieri ... Laura D. Locati
Published: December 31, 2021 Explor Target Antitumor Ther. 2021;2:522–542
This article belongs to the special issue Immunotherapy in Cancer Patients

