Open Access
Correction
Correction: Familial achalasia isolated or syndromic: about 18 families
Editorial Office
Published: January 11, 2024 Explor Dig Dis 2024;3:1
Open Access
Review
Liver injury related to Japanese herbal medicines: clinical features and diagnosis
Naoki Mantani
Published: June 27, 2023 Explor Dig Dis. 2023;2:77–82
This article belongs to the special issue Drug-induced Liver Injury: From Bench to Clinical Application
Open Access
Review
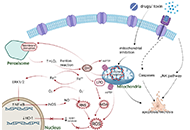
Role of oxidative stress and endoplasmic reticulum stress in drug-induced liver injury
Hanghang Wu ... Francisco Javier Cubero
Published: June 28, 2023 Explor Dig Dis. 2023;2:83–99
This article belongs to the special issue Drug-induced Liver Injury: From Bench to Clinical Application
Open Access
Original Article
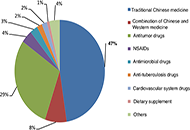
An analysis on the clinical features and risk factors associated with the prognosis of patients with drug-induced liver injury
Qian Wei ... Jinsheng Guo
Published: June 30, 2023 Explor Dig Dis. 2023;2:100–117
This article belongs to the special issue Drug-induced Liver Injury: From Bench to Clinical Application
Open Access
Review
Alcohol-related liver disease: also a question of what you drink?
Finn Jung ... Ina Bergheim
Published: June 30, 2023 Explor Dig Dis. 2023;2:118–132
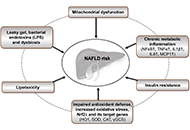
Open Access
Review
Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease—What are the proposed mechanisms?
Franziska A. Hägele ... Anja Bosy-Westphal
Published: August 24, 2023 Explor Dig Dis. 2023;2:133–148
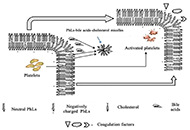
Open Access
Review
Pathophysiology of biochemical signs of primary biliary cholangitis
Vasiliy Ivanovich Reshetnyak, Igor Veniaminovich Maev
Published: August 27, 2023 Explor Dig Dis. 2023;2:149–171
This article belongs to the special issue CHOLESTASIS
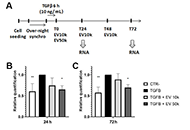
Open Access
Original Article
Human liver stem cell-derived extracellular vesicles modulate long non-coding RNA expression profile in an in vivo model of non-alcoholic steatohepatitis
Giulia Chiabotto ... Stefania Bruno
Published: August 30, 2023 Explor Dig Dis. 2023;2:172–187
Open Access
Review
Principles of risk stratification in nonalcoholic fatty liver disease. A narrative review emphasizing non-invasive strategies
Amedeo Lonardo
Published: August 30, 2023 Explor Dig Dis. 2023;2:188–201
This article belongs to the special issue Advances in Hepato-gastroenterology: Diagnosis, Prognostication, and Disease Stratification
Open Access
Review
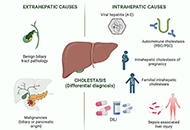
Drug-induced cholestasis: causative agents and challenges in diagnosis and management
Jose M. Pinazo-Bandera ... Miren García-Cortés
Published: September 18, 2023 Explor Dig Dis. 2023;2:202–222
This article belongs to the special issue CHOLESTASIS
Open Access
Review
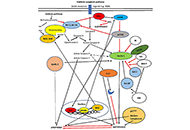
Interplay of autophagy, apoptosis, and senescence in primary biliary cholangitis
Elias Kouroumalis ... Argyro Voumvouraki
Published: October 16, 2023 Explor Dig Dis. 2023;2:223–245
This article belongs to the special issue CHOLESTASIS
Open Access
Editorial
Exploration of Digestive Diseases, where discovery and communication meet
Jose C. Fernandez-Checa
Published: July 01, 2022 Explor Dig Dis. 2022;1:1–3
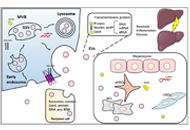
Open Access
Review
Extracellular vesicles in metabolic dysfunction associated fatty liver disease: mechanisms, diagnostic and therapeutic implications
Zongmei Wu ... Han Moshage
Published: July 13, 2022 Explor Dig Dis. 2022;1:4–20
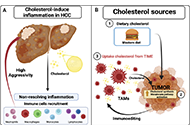
Open Access
Review
Tumor immune microenvironment modulation by cholesterol in hepatocellular carcinoma
Alejandro Escobedo-Calvario ... María Concepción Gutiérrez-Ruíz
Published: July 29, 2022 Explor Dig Dis. 2022;1:21–39

Open Access
Original Article
The hepatocyte growth factor induces an anti-inflammatory and repairing response in the cholestasis-induced colon damage
Jocelyn López-Ramirez ... Leticia Bucio-Ortiz
Published: August 11, 2022 Explor Dig Dis. 2022;1:40–50
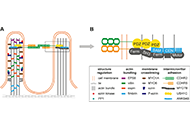
Open Access
Review
Fructose, a trigger of metabolic diseases?—a narrative review
Anja Baumann ... Ina Bergheim
Published: August 29, 2022 Explor Dig Dis. 2022;1:51–71
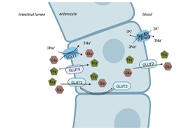
Open Access
Perspective
The intermicrovillar adhesion complex in gut barrier function and inflammation
Bernadette Mödl ... Robert Eferl
Published: October 11, 2022 Explor Dig Dis. 2022;1:72–79
Open Access
Review
Caspase-2 in liver disease and hepatocellular carcinoma
Amaya Lopez-Pascual ... Maite G. Fernández-Barrena
Published: October 31, 2022 Explor Dig Dis. 2022;1:80–96
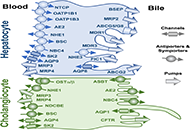
Open Access
Review
Etiopathogenesis and pathophysiology of cholestasis
Maitane Asensio ... Jose J. G. Marin
Published: October 31, 2022 Explor Dig Dis. 2022;1:97–117
This article belongs to the special issue CHOLESTASIS
Open Access
Original Article
Probiotic human alcohol dehydrogenase-4 expressing bacteria protects from diet-induced obesity and metabolic impairment: a new concept of disease prevention
Rajnish Prakash Singh ... Zvi Hayouka
Published: October 31, 2022 Explor Dig Dis. 2022;1:118–136

