Open Access
Commentary
Further arguments in favor of the biological relevance of purinergic receptors: the novel emergence of a purinergic signature
Cinzia Volonté, Rafael Franco
Published: November 29, 2023 Explor Neuroprot Ther. 2023;3:429–434
Open Access
Review
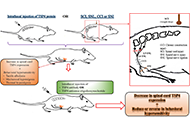
Blockage of thrombospondin 4 secreted by spinal astrocytes may be a promising therapeutic target in the treatment of neuropathic pain
Neslihan Düzenli ... Aytül Önal
Published: October 31, 2022 Explor Neuroprot Ther. 2022;2:226–241

Open Access
Editorial
Science plus technology to address challenges in determining the efficacy of neuroprotective/neurorestorative therapies
Rafael Franco
Published: August 05, 2021 Explor Neuroprot Ther. 2021;1:1–6

Open Access
Retraction
Retraction: Diffusion magnetic resonance imaging-based surrogate marker in amyotrophic lateral sclerosis
Editorial Office
Published: December 29, 2023 Explor Neuroprot Ther. 2023;3:513
Open Access
Original Article
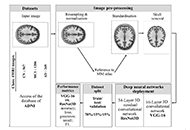
Alzheimer’s disease detection from magnetic resonance imaging: a deep learning perspective
Karolina Armonaite ... Luigi Laura
Published: June 30, 2023 Explor Neuroprot Ther. 2023;3:139–150
This article belongs to the special issue The Urgent Need for New Hypotheses to Develop Effective Therapeutic Tools Against Alzheimer's Disease
Open Access
Review
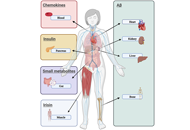
Looking at the periphery—new hypothesis to look for new targets for Alzheimer’s disease therapy
Jesús Avila ... Félix Hernández
Published: June 30, 2023 Explor Neuroprot Ther. 2023;3:151–163
This article belongs to the special issue The Urgent Need for New Hypotheses to Develop Effective Therapeutic Tools Against Alzheimer's Disease

Open Access
Review
Muscle fatigue and exercise-related biomarkers in amyotrophic lateral sclerosis
Francesca Bianchi ... Gabriele Siciliano
Published: June 30, 2023 Explor Neuroprot Ther. 2023;3:164–176
Open Access
Case Report
Tension pneumocephalus as a complication of surgical evacuation of chronic subdural hematoma: case report and literature review
Mohammed A. Azab ... Brandon Lucke-Wold
Published: August 23, 2023 Explor Neuroprot Ther. 2023;3:177–185
This article belongs to the special issue Emerging Concepts in Subarachnoid Hemorrhage
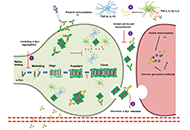
Open Access
Review
Targeting α-synuclein aggregation with immunotherapy: a promising therapeutic approach for Parkinson’s disease
Gabriela Henriquez, Mahesh Narayan
Published: August 25, 2023 Explor Neuroprot Ther. 2023;3:207–234
This article belongs to the special issue Parkinsons Disease: Principal Targets and Interventional Mechanisms

Open Access
Review
Retracted: Diffusion magnetic resonance imaging-based surrogate marker in amyotrophic lateral sclerosis
Yuya Saito
Published: August 25, 2023 Explor Neuroprot Ther. 2023;3:186–206
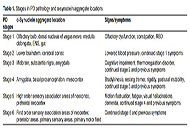
Open Access
Review
Rehabilitation for non-motor symptoms for patients with Parkinson’s disease from an α-synuclein perspective: a narrative review
Zhaoyang Liu ... Wen Liu
Published: August 27, 2023 Explor Neuroprot Ther. 2023;3:235–257
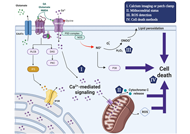
Open Access
Systematic Review
Various facets of excitotoxicity
Talita Glaser ... Henning Ulrich
Published: February 23, 2022 Explor Neuroprot Ther. 2022;2:36–64
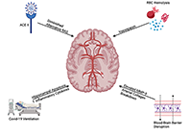
Open Access
Review
Subarachnoid hemorrhage: management considerations for COVID-19
Eric J. Panther, Brandon Lucke-Wold
Published: March 02, 2022 Explor Neuroprot Ther. 2022;2:65–73
This article belongs to the special issue Emerging Concepts in Subarachnoid Hemorrhage
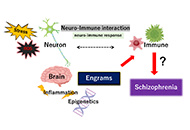
Open Access
Perspective
Gut microbiota could modulate the effects of neuro-immune responses and memory traces via the gut-brain-immune axis in schizophrenia
Haruka Sawamura ... Satoru Matsuda
Published: April 24, 2022 Explor Neuroprot Ther. 2022;2:74–86
This article belongs to the special issue Intervention of Neuroimmune Responses

Open Access
Review
The role of physical activity against chemotherapy-induced peripheral neuropathy: a narrative review
Daniele Diotti ... Lucio Marinelli
Published: April 28, 2022 Explor Neuroprot Ther. 2022;2:87–99

Open Access
Original Article
Combined effects of dry needling and exercises therapy on muscle spasticity and motor function in chronic stroke: a pretest-posttest pilot study
Seyedeh Saeideh Babazadeh-Zavieh ... Korosh Mansoori
Published: June 20, 2022 Explor Neuroprot Ther. 2022;2:100–109
This article belongs to the special issue Dry Needling for Neurological Disorders
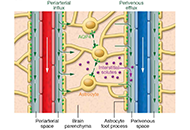
Open Access
Perspective
Can meditation-based approaches improve the cleansing power of the glymphatic system?
Peter Wostyn, Piet Goddaer
Published: June 20, 2022 Explor Neuroprot Ther. 2022;2:110–117
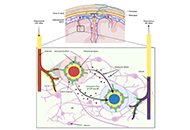
Open Access
Review
The glymphatic system and subarachnoid hemorrhage: disruption and recovery
Stephan Quintin ... Brandon Lucke-Wold
Published: June 21, 2022 Explor Neuroprot Ther. 2022;2:118–130
This article belongs to the special issue Emerging Concepts in Subarachnoid Hemorrhage

Open Access
Review
Economics of dry needling and botulinum toxin type A for treatment of post-stroke spasticity: a review
Daniel Fernández ... Eva María Gómez-Trullén
Published: June 30, 2022 Explor Neuroprot Ther. 2022;2:131–140
This article belongs to the special issue Dry Needling for Neurological Disorders
Open Access
Review
Autism: genetics, environmental stressors, maternal immune activation, and the male bias in autism
Sarah Otaru, David A. Lawrence
Published: August 11, 2022 Explor Neuroprot Ther. 2022;2:141–161
This article belongs to the special issue Intervention of Neuroimmune Responses

