Impact of iodinated contrast media on X-ray-induced DNA damage: a comprehensive review
Drawing insights from a spectrum of
in vitro,
in vivo experimental, and clinical studies, this review illuminates the underlying mechanism by which iodinated contrast media (ICM) exerts an indirect
[...] Read more.
Drawing insights from a spectrum of in vitro, in vivo experimental, and clinical studies, this review illuminates the underlying mechanism by which iodinated contrast media (ICM) exerts an indirect genotoxic effect. The mechanism involves the photoelectric effect induced by iodine molecules, thereby augmenting radiation attenuation and subsequently elevating the locally absorbed radiation dose. The ensuing generation of secondary electrons from each photoelectric absorption interaction triggers molecular reactions, culminating in discernible DNA damage, notably in the form of DNA double-strand breaks. A convergence of evidence from in vitro, experimental, and clinical investigations underscores a consistent pattern: the addition of iodine contrast linearly heightens the absorbed radiation dose and associated DNA damage. This quantification was evident through alterations in attenuation and the manifestation of double-strand breaks in circulating lymphocytes, serving as an intermediate endpoint and a potential long-term indicator of cancer. The observed surplus of DNA damage in contrast-enhanced images compared to non-contrast images ranged notably from +30% to +200%. This broad range accentuates a substantial amplification effect on radiation-induced damage, particularly noteworthy at clinically relevant iodine doses. Crucially, this effect remains unaffected by brands or manufacturers and exhibits a robust, exclusive correlation with the concentration of iodine in the bloodstream. The significant augmentation of absorbed dose and genotoxic impact of X-rays due to the use of contrast agents warrants critical attention within the medical community. This often-unacknowledged genotoxic influence may play a pivotal role in elevating cancer risks among patients undergoing radiation-based procedures, necessitating a reconsideration of risk assessment protocols and clinical practices.
Chiara Iacconi ... Enrica Ciofini
View:1370
Download:29
Times Cited: 0
Drawing insights from a spectrum of in vitro, in vivo experimental, and clinical studies, this review illuminates the underlying mechanism by which iodinated contrast media (ICM) exerts an indirect genotoxic effect. The mechanism involves the photoelectric effect induced by iodine molecules, thereby augmenting radiation attenuation and subsequently elevating the locally absorbed radiation dose. The ensuing generation of secondary electrons from each photoelectric absorption interaction triggers molecular reactions, culminating in discernible DNA damage, notably in the form of DNA double-strand breaks. A convergence of evidence from in vitro, experimental, and clinical investigations underscores a consistent pattern: the addition of iodine contrast linearly heightens the absorbed radiation dose and associated DNA damage. This quantification was evident through alterations in attenuation and the manifestation of double-strand breaks in circulating lymphocytes, serving as an intermediate endpoint and a potential long-term indicator of cancer. The observed surplus of DNA damage in contrast-enhanced images compared to non-contrast images ranged notably from +30% to +200%. This broad range accentuates a substantial amplification effect on radiation-induced damage, particularly noteworthy at clinically relevant iodine doses. Crucially, this effect remains unaffected by brands or manufacturers and exhibits a robust, exclusive correlation with the concentration of iodine in the bloodstream. The significant augmentation of absorbed dose and genotoxic impact of X-rays due to the use of contrast agents warrants critical attention within the medical community. This often-unacknowledged genotoxic influence may play a pivotal role in elevating cancer risks among patients undergoing radiation-based procedures, necessitating a reconsideration of risk assessment protocols and clinical practices.
 Impact of iodinated contrast media on X-ray-induced DNA damage: a comprehensive reviewOpen AccessReviewDrawing insights from a spectrum of in vitro, in vivo experimental, and clinical studies, this review illuminates the underlying mechanism by which iodinated contrast media (ICM) exerts an indirect [...] Read more.Chiara Iacconi ... Enrica CiofiniPublished: April 19, 2024 Explor Cardiol. 2024;2:79–87
Impact of iodinated contrast media on X-ray-induced DNA damage: a comprehensive reviewOpen AccessReviewDrawing insights from a spectrum of in vitro, in vivo experimental, and clinical studies, this review illuminates the underlying mechanism by which iodinated contrast media (ICM) exerts an indirect [...] Read more.Chiara Iacconi ... Enrica CiofiniPublished: April 19, 2024 Explor Cardiol. 2024;2:79–87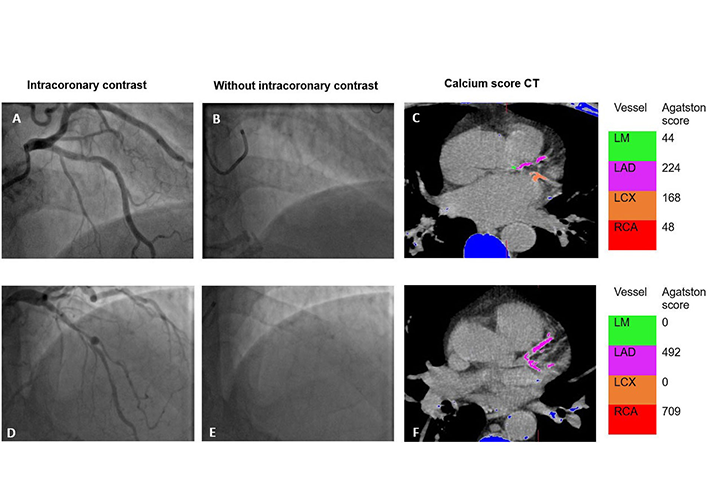 Coronary calcification on invasive angiography and the Agatston score—a single-center experienceOpen AccessOriginal ArticleAim: The pattern and severity of coronary artery calcification (CAC) can influence prognosis and outcome in percutaneous coronary intervention. An objective assessment of CAC during invasive angi [...] Read more.Zafraan Zathar ... Vinoda SharmaPublished: April 15, 2024 Explor Cardiol. 2024;2:67–78
Coronary calcification on invasive angiography and the Agatston score—a single-center experienceOpen AccessOriginal ArticleAim: The pattern and severity of coronary artery calcification (CAC) can influence prognosis and outcome in percutaneous coronary intervention. An objective assessment of CAC during invasive angi [...] Read more.Zafraan Zathar ... Vinoda SharmaPublished: April 15, 2024 Explor Cardiol. 2024;2:67–78 Nanoparticles loaded with the DNA methyltransferase inhibitor SGI-1027 decrease murine atherosclerosis and inflammation in cultured human macrophagesOpen AccessOriginal ArticleAim: The DNA of the atheroma is hypermethylated relative to adjacent healthy vascular tissue. A significant portion of hypermethylated loci in the atheroma DNA map to genes related to macrophage [...] Read more.Ana Cristina Márquez-Sánchez ... Silvio ZainaPublished: April 10, 2024 Explor Cardiol. 2024;2:49–66
Nanoparticles loaded with the DNA methyltransferase inhibitor SGI-1027 decrease murine atherosclerosis and inflammation in cultured human macrophagesOpen AccessOriginal ArticleAim: The DNA of the atheroma is hypermethylated relative to adjacent healthy vascular tissue. A significant portion of hypermethylated loci in the atheroma DNA map to genes related to macrophage [...] Read more.Ana Cristina Márquez-Sánchez ... Silvio ZainaPublished: April 10, 2024 Explor Cardiol. 2024;2:49–66 Role of cardio-ankle vascular index as a predictor of left ventricular hypertrophy in the evaluation of pediatric hypertensionOpen AccessOriginal ArticleAim: Cardio-ankle vascular index (CAVI) is a marker of arterial stiffness independent of blood pressure (BP) at the time of measurement. This work sought to evaluate the association of CAVI with [...] Read more.Evan Harvey ... Ranjit PhilipPublished: April 06, 2024 Explor Cardiol. 2024;2:40–48
Role of cardio-ankle vascular index as a predictor of left ventricular hypertrophy in the evaluation of pediatric hypertensionOpen AccessOriginal ArticleAim: Cardio-ankle vascular index (CAVI) is a marker of arterial stiffness independent of blood pressure (BP) at the time of measurement. This work sought to evaluate the association of CAVI with [...] Read more.Evan Harvey ... Ranjit PhilipPublished: April 06, 2024 Explor Cardiol. 2024;2:40–48