Affiliation:
1Emeritus Professor, Department of Dermatology, Kyushu University, Higashiku, Fukuoka, 812-8582, Japan
Email: furuemasutaka00@yahoo.co.jp
ORCID: https://orcid.org/0000-0002-2967-1073
Explor Immunol. 2021;1:37–47 DOl: https://doi.org/10.37349/ei.2021.00005
Received: February 04, 2021 Accepted: March 23, 2021 Published: April 30, 2021
Academic Editor: Lorenzo Cosmi, University of Florence, Italy
The article belongs to the special issue Cross Talk Among Skin Cells and Immune Cells
Interleukin (IL)-22 is produced from immune cells such as T helper (Th)22 cells, Th17/22 cells, and group 3 innate lymphoid cells. IL-22 signals via the IL-22 receptor 1 (IL-22R1) and the IL-10 receptor 2 (IL-10R2). As the IL-22R1/IL-10R2 heterodimer is preferentially expressed on border tissue between the host and the environment, IL-22 is believed to be involved in border defense. Epidermal keratinocytes are the first-line skin barrier and express IL-22R1/IL-10R2. IL-22 increases keratinocyte proliferation but inhibits differentiation. Aryl hydrocarbon receptor (AHR) is a chemical sensor and an essential transcription factor for IL-22 production. In addition, AHR also upregulates the production of barrier-related proteins such as filaggrin in keratinocytes, suggesting a pivotal role for the AHR-IL-22 axis in regulating the physiological skin barrier. Although IL-22 signatures are elevated in atopic dermatitis and psoriasis, their pathogenic and/or protective implications are not fully understood.
Interleukin (IL)-22 belongs to the IL-10-related cytokine family, which includes IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28 and IL-29 [1–3]. There is 79% homology between human and murine IL-22, and their respective genes are located on the same chromosome as interferon-γ (IFN-γ) [1–3]. The IL-22 receptor (IL-22R) is composed of a heterodimer of IL-22R1 and IL-10R2. The former protein is shared with the IL-20 and IL-24 receptor, while the latter is a component of the receptor for IL-10, IL-26, IL-28, and IL-29 [1–3].
Chronic inflammatory skin diseases such as atopic dermatitis and psoriasis bring about significant psychophysical and socioeconomic burdens to afflicted patients [4–8]. Recent therapeutic progress using biologics has demonstrated a critical pathogenic role for IL-4/IL-13-producing type 2 T helper (Th2) cells in atopic dermatitis and IL-17A-producing Th17 cells in psoriasis [4–8]. In addition to these essential axes, increased IL-22 signatures have been shown both in atopic dermatitis and psoriasis [9–14]. IL-22 is produced from specific acquired and innate hematopoietic cells, but its receptor, IL-22R1/IL-10R2, is preferentially expressed on non-hematopoietic cells such as epidermal keratinocytes [1–3]. Therefore, the physiological and pathological interaction between IL-22 and keratinocytes has gained particular attention from the viewpoint of skin barrier integrity and as a potential new target for the treatment of these inflammatory skin diseases [15, 16].
IL-22 is primarily produced by immune cells including CD4+ Th cells, CD8+ cytotoxic T (Tc) cells, natural killer T (NKT) cells, and group 3 innate lymphoid cells (ILC3) [1–3]. Non-lymphoid cells, including macrophages, neutrophils, mast cells, and fibroblasts may also produce IL-22, but production in keratinocytes does not occur [1–3, 15, 17]. Th and Tc cells are subdivided into several specialized subsets depending on surface markers, cytokine production and the expression of critical transcription factors as exemplified in Table 1 [18].
T cell subsets
| T cell subsets | Surface markers | Cytokine production | Gene expression of critical transcription factors |
|---|---|---|---|
| Th1/Tc1 | CXCR3 | IFN-γ | TBX21 |
| Th2/Tc2 | CCR4 | IL-4, IL-13, IL-5 | GATA3 |
| Th17/Tc17 | CCR4, CCR6 | IL-17A, IL-17F, IL-22 | RORC |
| Th17 + 1/Tc17 + 1 | CXCR3, CCR6 | IL-17A, IL-17F, IFN-γ | RORC, TBX21 |
| Th22/Tc22 | CCR4, CCR6, CCR10 | IL-22, TNF-α | AHR |
| Tfh/Tfc | CXCR5 | IL-21 | BCL6 |
| CD4+Treg/CD8+Treg | CCR2, CCR4 | IL-10, TGF-β | FOXP3 |
Tfh: T follicular helper; Tfc: T follicular cytotoxic; Treg: regulatory T; TGF: transforming growth factor
Among them, IL-22 is preferentially produced by Th22, ILC3, Th17 and to a lesser extent Tc22 and Tc17 cells [1–3, 18]. Although most IL-22-producing cells consist of IL-17A coproducing Th17/22 cells in mice, human IL-22 high-producers consist of Th22 cells that do not co-express IL-17A [1, 15, 19, 20]. Additionally, although human Th17 cells express CD161, Th22 cells do not [21]. ILCs, lacking antigen-specific T or B cell receptors, are divided into 4 subsets including ILC1 producing IFN-γ, ILC2 producing IL-13 and IL-5, ILC3 producing IL-17A and IL-22, and ILCreg producing IL-10 and TGF-β [1–3, 22, 23]. Almost all IL-22-producing cells, including ILC3, express CCR6, which recognizes only CCL20 (Table 1) [18, 24, 25]. Keratinocytes are a rich source of CCL20 [26, 27]; therefore, the CCL20/CCR6 axis may be important for the recruitment of Th22 cells in inflammatory skin diseases similar to that of Th17 cells [25, 28].
It is known that IL-22 production essentially depends on IL-23 [29, 30] and the aryl hydrocarbon receptor (AHR) [21, 31–33]. IL-23 (p19/p40) binds the IL-23R/IL-12Rβ1 heterodimer and activates the Janus kinase 2/tyrosine kinase 2 (JAK2/TYK2) and signal transducer and activator of transcription 3 (STAT3) pathway [34]. The IL-23-JAK2/TYK2-STAT3 axis appears to be crucial for IL-22 production in mice [29, 30], but may be dispensable in humans, as an IL-23 blockade profoundly decreased IL-17A but not IL-22 production [21].
AHR is a chemical sensor for various endogenous and exogenous ligands and serves as a cardinal transcription factor that promotes epidermal differentiation and barrier function [35–37]. The skin and intestinal tract are rich in AHR ligands produced from commensal microbiomes [32, 38–41]. Ultraviolet B (UVB) ray irradiation also generates high-affinity AHR ligands from tryptophan in the skin [42]. These AHR ligands are crucial for maturation of the host immune system against symbiotic commensal microbiomes via IL-22 induction [43]. In humans, AHR agonists reduce gene expression of the Th17 master transcription factor RORC without affecting TBX21, GATA3 and FOXP3 [21]. They also decrease the expression of IL-23R [21]. Importantly, AHR ligation not only decreases the number of Th17 cells but also primes naive CD4+ T cells to produce IL-22 without affecting IL-17A or IFN-γ production, suggesting a pivotal role of AHR in developing Th22, but not Th17, cells in humans [21, 31] (Figure 1). In contrast, development of both Th17 and Th22 cells is compromised in Ahr-deficient mice [33]. The number of IL-22-expressing ILCs is also markedly decreased in Ahr-deficient mice [32]. In addition to their potent activity towards Th22-prone immune deviation, AHR ligands can potentially upregulate the production of barrier-related proteins including filaggrin and loricrin, which enhance skin barrier integrity [35–37] (Figure 1).
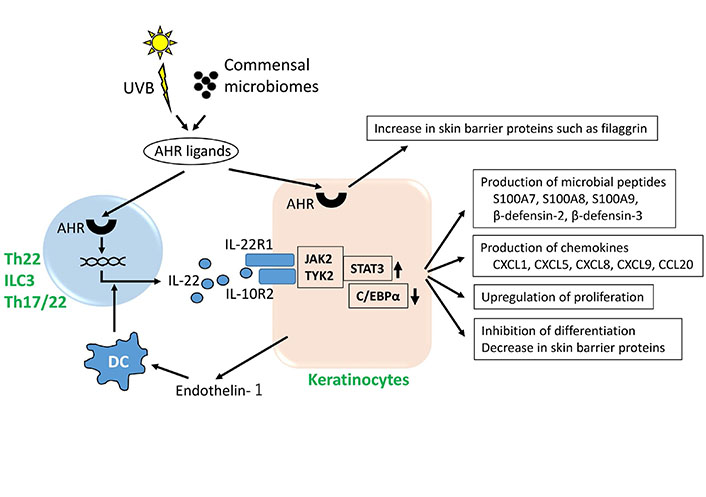
AHR, IL-22 and keratinocytes. IL-22 is produced by Th22 cells, Th17/22 cells and ILC3. UVB irradiation and commensal microbiomes generate various AHR ligands. AHR activation upregulates gene expression of IL-22 and also stimulates keratinocytes to increase production of barrier-related proteins such as filaggrin. Dendritic cells (DCs) treated with keratinocyte-derived endothelin-1 induce T cells to produce IL-22. Keratinocytes express IL-22R1 and IL-10R2 complex. IL-22 binds the IL-22R1/IL-10R2 heterodimer, activates the JAK2/TYK2 and STAT3 pathway and inhibits the activity of CCAAT/enhancer binding protein α (C/EBPα), stimulating keratinocytes to produce microbial peptides and chemokines. IL-22 upregulates proliferation and inhibits differentiation of keratinocytes
It is intriguing that AHR is also an essential upstream transcription factor for IL-24 production in keratinocytes [44–46]. The relationship between AHR activation and IL-20 production is unknown thus far.
IL-4 and IL-13 are critical in the pathogenesis of atopic dermatitis [36], disrupting the barrier function of epidermal keratinocytes by downregulating the production of barrier-related proteins such as filaggrin and loricrin [37]. Barrier-disrupted keratinocytes produce large amounts of thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 [47–49]. These cytokines stimulate DCs to induce Th2-prone T cell differentiation [47, 50, 51]. Although this Th2-prone vicious cycle is predominantly active in atopic dermatitis [36], the lesional skin of atopic dermatitis patients harbors varying numbers of Th22, Th17 and Th1 cells, thus exhibiting marked endotype heterogeneity [52].
A possible explanation for Th22, Th17 and Th1 cell induction in atopic dermatitis is endothelin 1. Endothelin 1 is constitutively produced by keratinocytes [53]. Physiologically, it is preferentially expressed in basal keratinocytes [54, 55], but it is overexpressed to a variable extent in inflamed epidermis [54, 56]. Intriguingly, it is pruritogenic and induces pruritus in mice as well as humans [57]. As described above, DCs treated with TSLP, IL-25, or IL-33 induce Th2-dominant immune response [47, 50, 51]. In sharp contrast, DCs treated with endothelin 1 prompt T cells to differentiate towards Th22, Th17 and Th1 lineages [56]. Moreover, endothelin 1 inhibits Th2 cell differentiation [56]. Thus, endothelin 1 is one of the cutaneous factors promoting IL-22 production [56, 58]. In line with this notion, topical application of endothelin receptor antagonist alleviates not only mite-induced dermatitis [59] but also imiquimod-induced psoriasiform skin inflammation [60]. Notably, there is a mutual feedforward regulatory circuit between IL-25 and endothelin 1—IL-25 upregulates the expression of endothelin 1, while endothelin 1 also upregulates the production of IL-25 in keratinocytes [54].
IL-22 binds the IL-22R1/IL-10R2 complex [1–3] and stimulates the JAK2/TYK2 and STAT3 pathway [34]. Unlike other members of the IL-10 cytokine family, IL-22 has a soluble secreted receptor, the IL-22 binding protein (IL-22BP) [61–63]. IL-22BP exhibits a much higher affinity for IL-22 than IL-22R1 and therefore prevents the binding of IL-22 to IL-22R1 [64, 65]. DCs and T cells can produce IL-22BP [66, 67], while keratinocytes are a much richer source of functional IL-22BP [61]. Deficiency in IL-22BP aggravates skin inflammation [61].
IL-20, IL-22 and IL-24 use IL-22R1 for their receptor complexes [1–3]. Although IL-22 transmits signals via IL-22R1/IL-10R2, IL-20 and IL-24 can signal via IL-22R1/IL-20R2 as well as IL-20R1/IL-20R2 [68]. IL-20R2 and IL-10R2 are consistently expressed on the surface of cultured human keratinocytes regardless of confluence, passage number, or calcium levels in the medium [68]. In contrast, surface expression of both IL-20R1 and IL-22R1 is low in monolayer culture, and becomes high in 3-dimensional reconstituted human epidermis [68]. When IL-22R1-overexpressed keratinocytes are treated with 10 ng/ml of IL-20, IL-22 and IL-24, IL-22 induces the production of CCL20, CXCL8 and heparin-binding epidermal growth factor-like growth factor (HB-EGF) more potently than IL-20 and IL-24 [69]. Although T cells, B cells, NK cells and monocytes do not express IL-20R1 and IL-22R1 [68], functional IL-22R1 is known to be expressed on T cells from anaplastic lymphoma kinase-positive anaplastic large cell lymphoma patients [70].
IL-6 plays a critical role in the expression of IL-22R1 in keratinocytes because its expression is markedly decreased in IL-6-deficint mice [71]. MicroRNA-197 (miR-197) enhances the expression of IL-22R1 likely because it upregulates expression of the IL-6 receptor in keratinocytes [72, 73].
Many researchers have proposed a key role for IL-22 in epithelial border patrol especially in the intestinal tract, skin and airway [16, 74, 75]. The intestinal tract and its commensal and pathologic microbiomes maintain a homeostatic equilibrium with regard to host defense. IL-22 stimulates epithelial cells to produce antimicrobial peptides that are synergistically or additively upregulated in the presence of IL-17A [16]. IL-22 upregulates the production of CXCL1, CXCL5, CXCL9 and IL-6, which induce recruitment of relevant innate and acquired immune cells [16] (Figure 1). In addition, IL-22 induces the production of complement 3 from hepatocytes, which facilitates neutrophil killing of invading pathogens [74, 75]. Numerous AHR agonists are supplied to the intestinal tract from the diet and microbial metabolites which facilitate IL-22 production from intestinal IL-22-producing immune cells [76].
The skin is a body surface border, and epidermal keratinocytes are major cellular constituents of the host defense against the extracutaneous environment. UVB ray irradiation [42, 77], commensal microbiomes [40, 41] and environmental chemicals [78, 79] supply numerous AHR agonists to the skin. IL-22 stimulates keratinocytes to produce microbial peptides and chemokines such as S100A7, human β-defensin 2, human β-defensin 3 and CXCL8 [15, 16, 80–83] (Figure 1). However, the enhancing effect of IL-22 is relatively lower than other inflammatory cytokines [82, 84].
The expression of IL-22 is upregulated in their lesional skin of patients with atopic dermatitis and psoriasis [12–15]. IL-22 accelerates proliferation and migration of keratinocytes via STAT3 activation, and inhibits the terminal differentiation [69, 80, 85–87]. IL-22 blocks epidermal differentiation by inhibiting the expression of keratin 1 [80, 85, 86], keratin 10 [83, 88], involucrin [83, 86], loricrin [83, 88] and filaggrin [80, 83, 85, 87, 88]. In addition to STAT3 activation, IL-22-mediated downregulation of C/EBPα is also involved in the upregulation of proliferation and inhibition of differentiation in keratinocytes [89] (Figure 1). It is also known that IL-22- or IL-17A-treated keratinocytes increase their stemness by enhancing expression of CD29, CD44 and p63 [90].
House dust mites increase IL-22R1 expression and enhance the effects of IL-22 in keratinocytes [91]. UVB irradiation enhances the translocation of IL-22R1 from the cytosol to the membrane, and upregulates the responsiveness of keratinocytes to IL-22 [92]. IL-22 stimulates keratinocytes to produce IL-19, IL-20 and IL-24 [69]. IL-24 may also contribute to inhibit the expression of filaggrin via JAK1-STAT3 activation [69, 80, 93, 94] and to accelerate keratinocyte proliferation and S100A7 production [68].
Both IL-22 and IL-24 induce ROS production [95–97], while antioxidative AHR ligands may reduce the inflammatory action of IL-22 and IL-24. In fact, the antioxidant luteolin-7-glucoside alleviates ROS production and inhibits IL-22-mediated STAT3 activation [98].
However, IL-22 exhibits a beneficial effect on tight junctions. A recent study of bronchial epithelial cells demonstrated that IL-22 has the potential to reduce inflammation during influenza infection by enhancing tight junction activity [99]. Such protective function of IL-22 on tight junctions has been shown in keratinocytes in vitro, while IL-17A significantly downregulates tight junction expression in the epidermis [100].
IL-22 is produced from hematopoietic cells, and its receptor, IL-22R1/IL-10R2, is expressed on keratinocytes. Ligation of IL-22R1/IL-10R2 by IL-22 generally increases proliferation and inhibits differentiation of keratinocytes. This fundamental effect of IL-22 appears to work either as a pro- or anti-inflammatory depending on the type and timing of skin inflammation involved, but the precise physiopathological roles of IL-22 in the skin are not fully understood. Recent clinical studies have revealed that excess IL-22 in lesional skin may worsen atopic dermatitis, because the anti-IL-22 antibody fezakinumab shows a therapeutic potential for treating severe atopic dermatitis patients [11, 101]. Further clinical studies are necessary to explore the exact pathogenic implications of IL-22 in skin inflammation.
AHR: aryl hydrocarbon receptor
DCs: dendritic cells
IFN-γ: interferon-γ
IL: interleukin
IL-22BP: IL-22 binding protein
IL-22R: IL-22 receptor
ILC3: innate lymphoid cells
JAK2: Janus kinase 2
STAT3: signal transducer and activator of transcription 3
Tc: cytotoxic T
Th: T helper
TYK2: tyrosine kinase 2
UVB: ultraviolet B
Masutaka F wrote and Mihoko F revised the first draft. After English editing was performed, Masutaka F and Mihoko F agreed the final version and submitted the article.
The authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Yen Hai Vu ... Gaku Tsuji
Mariko Seishima ... Kuniaki Saito
Ichiro Katayama ... Mari Wataya-Kaneda
Kanami Orihara
Xinhui Ni, Yuping Lai
