Affiliation:
Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, FL 32611, USA
Affiliation:
Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, FL 32611, USA
Affiliation:
Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, FL 32611, USA
Affiliation:
Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, FL 32611, USA
Affiliation:
Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, FL 32611, USA
Affiliation:
Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, FL 32611, USA
Affiliation:
Department of Neurosurgery, College of Medicine, University of Florida, Gainesville, FL 32611, USA
Email: Brandon.Lucke-Wold@neurosurgery.ufl.edu
ORCID: https://orcid.org/0000-0001-6577-4080
Explor Neurosci. 2025;4:100695 DOl: https://doi.org/10.37349/en.2025.100695
Received: March 05, 2025 Accepted: May 09, 2025 Published: June 17, 2025
Academic Editor: Gabrio Bassotti, University of Perugia, Italy
The article belongs to the special issue Enteric Neuro-Gliopathies: Ready for Prime Time?
Spinal myelopathies, characterized by neurological deficits due to spinal compression in the spinal column, are increasingly common in the aging population. Although spinal myelopathies commonly present with sensory and motor deficits, they also manifest with life-debilitating enteric dysfunction associated with increased gastroparesis, constipation, bloating, abdominal pain, neurogenic bowel disease, and bladder and bowel incontinence. That said, the effects of spinal myelopathies on enteric gastrointestinal (GI) function are still poorly understood. This review aims to summarize existing literature concerning spinal myelopathies and their effect on the GI system, including the relevant anatomy and physiology of the nervous systems, etiology of various spinal cord injuries, clinical manifestations, current diagnosis and treatment strategies, and ongoing research concerning the gut-brain-spinal axis. The autonomic nervous system contributes to GI innervation through enteric reflex arcs and communication with the central nervous system (CNS) via spinal nerves. When spinal cord damage occurs, enteric reflex arcs, autonomic regulation, and gut-brain-spinal axis can become impaired, leading to GI symptoms. Etiologies of spinal myelopathies occur at all spinal levels. Spinal myelopathy includes inflammatory processes, such as multiple sclerosis and infection, and non-inflammatory processes, such as spondylosis, degenerative disc disease, tumors, and traumatic spinal cord injuries. Diagnosis modalities include imaging, particularly MRI, and functional assessments, such as high-resolution anorectal manometry and colonic transit studies. Enteric dysfunction treatment includes non-pharmacological, pharmacological, neuromodulatory interventions, and surgery. These strategies encompass lifestyle modifications, laxatives, prosecretory agents, 5HT4 agonists, vagus nerve stimulation, sympathetic nerve stimulation, colostomy, and ileostomy. Despite these treatment options, ongoing research with pudendal nerve stimulation, transanal irrigation, mesenchymal stem cells, and the relationship between the gut microbiome and gut-brain-spinal nerve axis may be beneficial in understanding spinal cord myelopathy-related enteric dysfunction, diagnosis, and treatment, ultimately improving clinical outcomes and quality of life for those who are affected.
Myelopathy, used interchangeably with spinal myelopathy, refers to any neurologic deficit related to the spinal cord [1, 2]. Spinal myelopathies are characterized by severe spinal cord compression occurring within the spinal column [3]. They can be further divided into two groups based on their etiology: inflammatory myelopathies, which include transverse myelitis, multiple sclerosis (MS), and viral myelitis, and non-inflammatory myelopathies, which include traumatic spinal cord injuries, tumors/epidural abscesses, and degenerative conditions such as cervical spondylotic myelopathy (CSM), among others [4]. Spinal myelopathies are becoming increasingly common in aging populations worldwide, with CSM alone accounting for the hospitalization of 15,000–20,000 patients per year in the United States [5]. Common clinical manifestations of spinal myelopathy include pain, extremity numbness, weakness, gait instability, paresthesia, dysesthesia, and, in severe cases, loss of bladder function [6–10]. With this wide range of clinical presentations, diagnosing spinal myelopathies can be challenging. A systematic approach incorporating a detailed physical examination, laboratory testing, advanced neuroimaging, and cerebrospinal fluid analysis is thus paramount to timely diagnosis and intervention [4, 6, 11].
Traditionally, spinal cord injury (SCI) and myelopathy have been described in association with motor and sensory deficits. An often overlooked and severe complication of myelopathy, however, lies in its ability to disrupt autonomic regulation, specifically control over the gastrointestinal (GI) tract. Acutely, spinal myelopathies have been associated with constipation and abdominal bloating [12, 13]. More chronic issues include abdominal pain, gastroparesis, and fecal incontinence as a result of neurogenic bowel [14–16]. Both contribute to poor quality of life among patient populations and are a large cause of hospitalization among people with SCI [16, 17]. The autonomic nervous system (ANS) is a branch of the peripheral nervous system that regulates various involuntary physiological processes, helping to maintain homeostasis [16, 18, 19]. It can be further divided into three divisions, all of which play essential roles in maintaining GI function: the sympathetic nervous system responsible for inhibiting GI activity (“fight or flight”), the parasympathetic nervous system responsible for stimulating GI activity (“rest and digest”), and the enteric nervous system (ENS) responsible for mediating digestive motor functions such as peristalsis and intestinal epithelial barrier functions including regulating gastric secretions [19–21]. All three divisions work in concert with the spinal cord via afferent and efferent nerve fibers, emphasizing the impact spinal myelopathies may have on enteric function.
The impact of spinal cord myelopathy on GI function continues to be poorly understood [17]. This review aims to summarize existing literature on how spinal cord damage may induce enteric dysfunction, incorporating discussion of relevant anatomy and paths of innervation. It focuses on the etiology of various spinal cord injuries, namely cervical myelopathy, thoracic myelopathy, lumbar/sacral myelopathy, and traumatic spinal cord injuries, as well as the associated symptoms that arise from these conditions.
Proper discussion of the effects of spinal myelopathy in enteric dysfunction necessitates reference to the role of spinal innervation in the maintenance of digestion. The GI tract heavily relies on the nervous system to facilitate proper digestion and absorption of food. It primarily receives innervation from the ANS and sensory divisions of the peripheral nervous system (Figure 1). The ANS consists of the sympathetic, parasympathetic, and enteric divisions, each playing an important role in GI function [22]. With regards to the extent of innervation of the GI tract, the ENS is the most prominent of the nervous system divisions, consisting of over 100 million neurons in humans [23]. The ENS has earned the name of the “second brain” on account of its autonomy and similarities to the brain [24]. A number of researchers have achieved full or partial separation of the ENS from the central nervous system (CNS) while still maintaining bowel function in mammals [25, 26]. However, pathologies, such as Hirschsprung disease and Chagas disease, which lead to impaired or missing portions of the ENS, can almost entirely halt proper GI functioning and be fatal [27]. Despite the autonomy of the ENS, spinal nerves still play an important role in providing feedback to the CNS and regulating digestion and defecation.
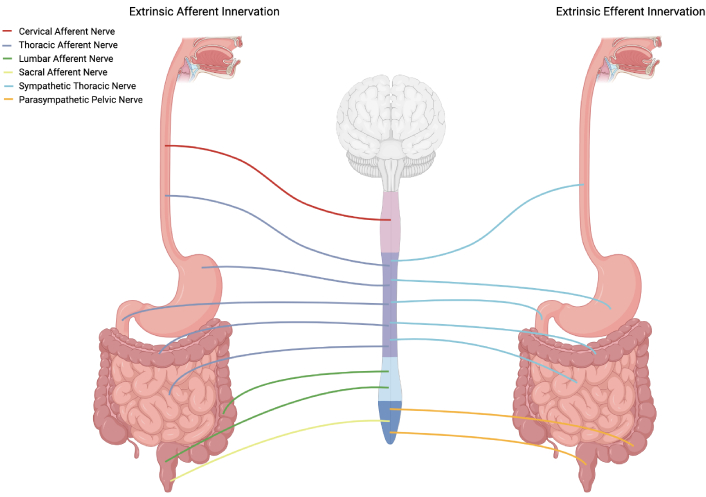
Innervation of the GI tract by spinal nerves. Paravertebral and prevertebral ganglia are not pictured. Exact spinal levels are not represented. Somatic innervation of the external anal sphincter by the pudendal nerve is not pictured. GI: gastrointestinal. Created in BioRender. Rahman, M. (2025) https://BioRender.com/g13i886
The ENS consists of a diversity of cell types of varying functional roles. Enteric neurons can be primarily grouped into motor neurons, interneurons, and intrinsic primary afferent neurons (IPANs) [28]. Motor neurons in the ENS control smooth muscle, mucosal secretions, endocrine function, vasodilation, and lymphatic tissue. These motor neurons can be inhibitory or excitatory. IPANs serve to monitor the state of the GI tract organs. Interneurons connect these two types of neurons allowing for reflexes that control GI function autonomously. The ENS is organized into two plexuses, namely the submucosal (formerly Meissner’s) plexus and myenteric (formerly Auerbach) plexus [29]. The submucosal plexus is primarily involved in controlling the vasculature of the GI tract as well as regulating stimulatory and absorptive activity of digestive enzymes and products. The submucosal plexus is well defined in the intestines but is sparsely present in the stomach and esophagus [28]. In contrast, the myenteric plexus consists of a singular layer of nervous tissue between the circular and longitudinal layers of smooth muscle in the GI tract. The primary role of the myenteric plexus lies in regulating the activity of smooth muscle throughout the GI tract. There are also a number of connections between the two plexuses and to other sources of innervation [29].
Spinal afferent innervation of the GI is carried out by specialized neurons designed to detect stimuli such as stroking of the mucosa or distension and contraction of the gut wall and mesentery and relay these signals to the CNS. Certain segments of the GI tract receive extrinsic afferent innervation from neurons of different spinal origins. Specifically, the upper portion of the GI tract, from the esophagus to the upper portion of the colon, receives afferent innervation from splanchnic nerves originating in the thoracolumbar segment of the spinal cord [30]. Additionally, the esophagus receives extrinsic afferent innervation from afferent neurons of cervical spinal origin [31]. While not of spinal origin, these upper portions of the GI tract are also innervated by vagal afferents [30]. The lower portion of the colon and the rectum instead receive afferent innervation by way of sacral and pelvic nerves of lumbosacral origin as well as afferents of thoracolumbar origin [30]. Afferent neurons of thoracolumbar origin have cell bodies residing in the dorsal root ganglia and often travel through prevertebral ganglia of sympathetic neurons where collaterals can synapse with other neurons [32]. The visceral pain as well as the resulting visceromotor reflex caused by colorectal distension is controlled by pelvic afferents of lumbosacral origin [32].
Extrinsic sympathetic innervation of the GI from nerves of thoracolumbar origin primarily acts upon ganglia of the ENS. Therefore, the primary targets of extrinsic sympathetic innervation are submucosal and myenteric ganglia. These neurons that act on submucosal and myenteric ganglia have cell bodies in the prevertebral ganglia [33]. Sympathetic stimulation of submucosal ganglia serves to inhibit secretomotor function of the ENS while sympathetic stimulation of the myenteric ganglia serves to inhibit GI motility [34]. In addition to the innervation of enteric ganglia, there is also direct extrinsic sympathetic innervation of the smooth muscle of sphincters and of arterioles throughout the GI tract [34]. The cell bodies of sympathetic neurons innervating the smooth muscle of sphincters are also present in the prevertebral ganglia, and these neurons serve to cause contraction of smooth muscle in sphincters [35]. In contrast, cell bodies of sympathetic neurons innervating GI arterioles are primarily localized in the paravertebral ganglia, and these neurons stimulate vasoconstriction [35, 36]. In sum, the extrinsic sympathetic innervation of the gut leads to an inhibition of digestion and movement of GI contents.
Extrinsic parasympathetic innervation of the upper GI tract is primarily controlled by the vagus nerve while the lower portions of the colon and rectum receive parasympathetic innervation by way of pelvic nerves from the sacral spinal cord [37]. These parasympathetic neurons act on enteric ganglia in a similar manner to their sympathetic counterparts and have cell bodies located in the hypogastric plexus. However, parasympathetic innervation of enteric ganglia instead leads to stimulation of colorectal motility [37]. Furthermore, parasympathetic nerves of sacral origin are involved in reflex activation of defecation in the distal colon and rectum triggered by distension of these areas [38].
While all of the previously discussed neural pathways in the process of digestion are not controlled somatically, there is additional innervation of the external anal sphincter muscles (should probably be muscle not plural) which can be somatically activated. While the internal anal sphincter is tonically contracted and is able to relax by way of the ENS when distention is present, the external anal sphincter receives extrinsic somatic innervation by way of the pudendal nerve of sacral origin [39]. Furthermore, somatic control of abdominal muscles contributes to the process of voluntary defecation [40]. It has also been suggested that increases in intra-abdominal pressure by way of abdominal muscle contraction could lead to stimulation of the ENS [40].
Spinal myelopathies can substantially alter GI function through disruption of spinal neural pathways that control autonomic and somatic nervous system function on the GI tract. While the ENS can maintain autonomous control over GI motility through intrinsic reflex arcs, disruption of extrinsic neural input can have pathological effects by way of impaired attenuation of ENS activity through myenteric and submucosal ganglia connections and impaired control of extrinsically innervated smooth muscle.
Damage to the spinal cord that affects the innervation of the GI tract by spinal nerves can result in neurogenic bowel, a condition characterized by alterations in GI innervation that leads to impaired function of the colon [41]. Lesions to the spinal cord, particularly supraconal lesions—those occurring above the conus medullaris—interrupt descending motor pathways and ascending sensory pathways, preventing parasympathetic inputs from the sacral spinal cord (S2–S4). Sacral parasympathetic activity regulates distal colonic motility and defecation reflexes. Patients with such lesions frequently present with prolonged colonic transit times, particularly in the descending colon and rectum, the region in which motility is most dependent on extrinsic innervation [42]. Sacral spinal reflex arcs allow reflexive bowel evacuation in response to rectal distension without requiring upper CNS control allowing for a preservation of some level of GI motility [38, 41, 43]. However, when there are upper motor neuron lesions, these reflexes can be hyperactive, causing a spastic bowel and sphincter responses [44]. In contrast, lower motor neuron lesions impair these reflex arcs, resulting in areflexia and flaccid bowel dysfunction [41, 44].
Autonomic dysfunction of the GI tract is characteristic of spinal myelopathies and arises from the disconnection of supraspinal control over sympathetic and parasympathetic outputs. Lesions above T6 level, which are superior to the greater splanchnic nerves, can cause autonomic dysreflexia which results from stimuli such as bowel distension, leading to increased sympathetic activation inferior to the lesion [45]. The heightened sympathetic activation causes severe hypertension through vasoconstriction, and, as a result of the increased systemic blood pressure, vascular baroreceptors signal compensatory parasympathetic activation to induce bradycardia and systemic vasodilation. However, impaired conduction of inhibitory parasympathetic signals through the injured spinal cord exacerbates the imbalance between sympathetic and parasympathetic responses preventing correction of excessive sympathetic activation below the spinal cord lesion, leading to potentially lethal complications [46].
As has been discussed, innervation is integral to the function of digestion and mobility through the GI tract. Therefore, damage to the spinal cord disrupts the autonomic innervation of the GI tract and can lead to chronic GI problems [47]. This section will explore the etiologies of spinal myelopathies, paying attention to the different spinal regions, and how these may cause varying degrees of impact on GI function (Figure 2).
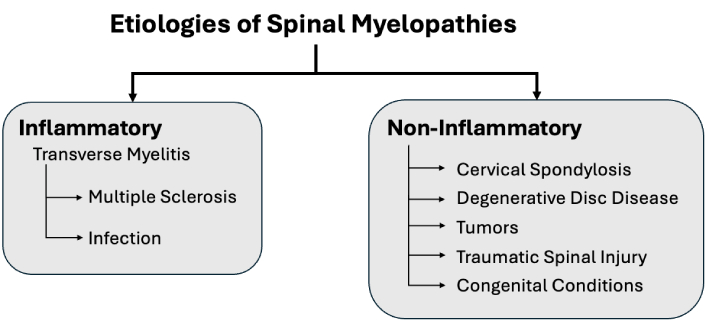
Beyond congenital conditions and cervical spondylotic myelopathy, there are common etiologies of spinal myelopathies for the levels of the spine. The cervical, thoracic, and lumbosacral levels of the spinal cord are subject to degeneration, trauma, inflammation, and tumors, resulting in gastrointestinal impact
CSM is the most common cause of myelopathy in adults, with peak incidence occurring between ages 40 and 60 [48, 49]. This condition arises from compression or inflammation of the spinal cord due to the gradual degeneration of the cervical vertebrae, intervertebral discs, and ligaments [50–54]. Factors contributing to this compression are characterized as static or dynamic [8]. Static factors include those that lead to stenosis of the central canal as well as general decline in the intervertebral discs and ligaments over time. Dynamic factors include repetitive movements that impact. As a result, the intervertebral discs no longer support weight.
In interviewing patients with CSM, Davies and colleagues [55] (2022) found that 12% of patients report fecal incontinence, thereby impacting GI function. In a separate study, Nouri and colleagues [56] (2020) found that 16% of the patients with degenerative cervical myelopathy reported GI complications. Interestingly, these patients were more frequently female and had a higher prevalence of psychiatric comorbidities, while also demonstrating less evidence of SCI on MRI [56]. Ng and colleagues [13] (2005) identified a higher likelihood of constipation in cervical injuries compared with lumbar injuries, whereas abdominal bloating was more likely in cervical and lumbar injuries compared with thoracic injuries. On the other hand, fecal incontinence and abdominal pain were not significantly associated with a specific level of injury [13]. Mehrotra and colleagues [57] (2017) observed no significant correlation between bowel complications and the affected spinal level. Surgical intervention for cervical myelopathy has shown mixed results. The study reported improvements in constipation, though outcomes were less favorable compared to dorsal myelopathy [57]. Conversely, a meta-analysis by Garg and Aggarwal [58] found no significant reduction in GI symptoms following decompressive surgery. Surgical intervention for treating CSM also poses risk for several post operative complications including dysphagia [59].
Thoracic myelopathies arise from various etiologies, including vertebral damage, osteophyte formation, dehydration of vertebral discs, and disc prolapse [60, 61]. Ossification of the ligamentum flavum (OLF) and posterior longitudinal ligaments (OPLL) can also lead to compression of the spinal cord and often requires surgical decompression to ameliorate pain and neurological symptoms [62]. Degenerative changes are less common in the thoracic spine due to the limited motion of the rib cage [63]. Among patients undergoing surgical decompression, only 4.3% exhibited thoracic stenosis from degenerative disease [64]. Tumor growth can also lead to spinal cord compression and, consequently, symptoms of myelopathy [65]. These tumors can be intramedullary or extramedullary in origin and can occur due to primary or metastatic growth [65]. Spinal meningiomas in particular, occurs frequently in the thoracic region [66]. Spinal cord tumors represent the most frequent cause of thoracic myelopathy (54%), followed by OLF (16%) and OPLL (10%) [63]. In the same study, approximately 9% of affected individuals reported bowel or bladder disturbances, with no significant associations between these bowel disturbances and disease etiology or thoracic spine level affected [63].
Lumbosacral myelopathy is less common due to termination of spinal cord at levels L1–L2. Damage to the T12–L2 region may result in conus medullaris syndrome (CMS), affecting the S1–S5 nerve roots and parasympathetic control of rectal sensation [67–69]. DDD was mentioned previously with cervical myelopathies; however, disc degeneration can also occur in the lumbar area as well and can contribute to lower back pain [70]. Due to variability in conus medullaris level location among human anatomy, CMS is not always clearly differentiated from CES [69]. Although anatomically distinct structures, CMS and CES are frequently concurrently described [69, 71]. CES is most commonly caused by disc pathology (45%), followed by tumors (29%), and infections (28%) [72]. Both syndromes manifest with micturition and defecation dysfunction [71, 73].
So far in this section, etiologies of myelopathies have been discussed by spinal cord level. However, there are a range of conditions that may cause myelopathy at any location. Myelopathy from transverse myelitis is caused by inflammation of the spinal cord [74]. In analyzing a group of 457 patients referred for transverse myelitis, Barreras and colleagues [74] (2018) found that only 54% were confirmed to have inflammatory myelopathy. Interestingly, bladder and bowel dysfunction were more commonly associated with vascular than inflammatory myelopathy (4.12 OR for vascular myelopathy). Transverse myelitis that causes inflammatory myelopathy, encompasses a broad range of mechanisms, including demyelinating, autoimmune, and infectious diseases [74, 75]. In a cohort of patients with transverse myelitis, the most common etiology was idiopathic (68.2%), followed by MS (16.4%) and infection (4.8%) [75]. Transverse myelitis can result in long-term bowel dysfunction in 77% of patients [76]. Severe complications, such as bowel obstruction and necrosis, have also been reported [77]. In a case study, a patient developed transverse myelitis, possibly due to enterovirus infection, which was then complicated by bowel obstruction, necrosis, and perforation, demonstrating the potential for fatal bowel dysfunction from transverse myelitis [77].
As discussed in the previous section, MS can lead to lesions that may compress the spinal cord when the immune system targets the myelin sheath in the white matter of the CNS. Neurogenic bowel is a prevalent disorder in patients with MS due to the impact on autonomic neural pathways controlling defecation [78]. It has been reported that anywhere between 39% to 73% of patients with MS experience symptoms including fecal incontinence and constipation [68]. Conversely, another study reported that 14.5% of patients with MS experience these symptoms, demonstrating that further research is required to adequately assess neurogenic bowel in patients with MS [79].
Traumatic spinal cord injuries, commonly caused by motor vehicle accidents, sports injuries, or violence, initiate ischemic and inflammatory cascades that exacerbate neuronal damage [80]. This damage is rarely reversible due to poor recovery of neurons and oligodendrocytes, leading to lifelong consequences [80]. In individuals with an injury above T6, damage to neural structures can lead to autonomic dysreflexia, that is characterized by episodes of elevated systolic blood pressure [46]. Bowel and bladder signals are the most common stimuli for these episodes [46]. Using a questionnaire in a group of 128 people with SCI, Liu and colleagues [81] (2009) found that 46.9% of SCI patients exhibited neurogenic bowel symptoms, namely constipation and fecal incontinence, significantly impacting their quality of life. Superior mesenteric artery syndrome (SMAS) may also present in these patients [82]. SMAS is not directly caused by the neurological effects of the injury but is instead believed to result from rapid weight loss following the trauma [82]. SMAS is characterized by compression of the duodenum by the superior mesenteric artery, which occurs due to a reduction in the surrounding mesenteric fat pad. Common symptoms include nausea and abdominal pain [82].
Congenital disorders that lead to myelopathy are less frequently described, most often appearing in selective case reports. Congenital conditions can include aplasia of the atlas, cervical stenosis, and spina bifida [83–85]. Congenital conditions lead to anomalies that are asymptomatic, however, in some cases may cause neurologic symptoms as reported [83, 84]. In such cases, surgical interventions may be used to manage the conditions. These conditions can also be associated with GI dysfunction. In a group of 395 patients with spina bifida, Brochard and colleagues [86] (2017) found that 61% of patients reported moderate or severe bowel dysfunction, as assessed by the neurogenic bowel dysfunction score.
As discussed, spinal myelopathies often present with enteric disturbances leading to significant clinical implications [87]. This section describes the spectrum of clinical presentations for enteric dysfunction associated with spinal myelopathies, highlighting their impact on patient outcomes and quality of life.
GI complications such as constipation, diarrhea, abdominal bloating, pain, dyspepsia, gastroparesis, and fecal incontinence are prevalent among patients with spinal myelopathies [56, 87] (Figure 3). Manifestations of GI complications are often linked to impaired autonomic regulation, which in turn adversely impacts GI motility [56]. This is particularly relevant to spinal myelopathies as GI issues can lead to malnutrition, anemia, and vitamin B12 deficiency, all of which have the potential to further exacerbate spinal cord dysfunction and negatively impact any potential myelopathy-related surgical recovery [56]. Case studies highlight the interplay between GI and neurological symptoms. A case study has described a patient with acute transverse myelitis following Campylobacter jejuni infection who initially presented with diarrhea but subsequently developed bowel dysfunction, lower limb weakness, and sensory disturbances, indicative of spinal cord involvement [88]. It must be emphasized that this case report identifies the significance of considering a potential neurological sequel in patients with acute GI symptoms who present with new-onset neurological symptoms. Early diagnosis and treatment continue to be crucial to improving patient outcomes and minimizing long-term neurological injury [88].
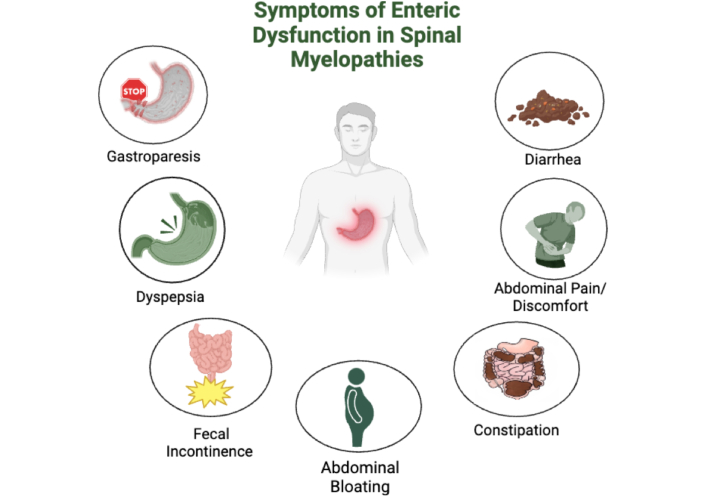
Gastrointestinal complications in patients with spinal myelopathies. Created in BioRender. Rahman, M. (2025) https://BioRender.com/z29w269
Mechanisms underlying these GI disturbances are complex and multifaceted. In a review of GI motility disorders in neurologic disease, Camilleri [87] (2021) emphasizes that disruptions in the ENS can lead to motor, sensory, storage, and excretory disruptions, manifesting as complex GI symptoms. Some of the symptoms listed include dysphagia, intestinal pseudo-obstruction, gastroparesis, diarrhea, and fecal incontinence. Furthermore, Camilleri [87] emphasizes that interplay between the central and ENS s is crucial in understanding these manifestations. Consequently, effective management of gut neuromuscular disorders involves not only addressing the underlying neurologic condition but also prioritizing the restoration of hydration and nutritional status [87].
The impact of autonomic dysfunction on GI symptoms in MS, a specific type of spinal myelopathy, has also been extensively reviewed [89]. Sirbu and colleagues [89] (2020), for example, documented a prevalence of GI symptoms ranging from 45% to 68% in patients with MS [90]. These symptoms, including dyspepsia and bowel irregularities, reflect disrupted communication between the central and ENS [89]. A retrospective study by Khanna and colleagues [91] (2022) further highlights the high prevalence of GI motility disorders in patients with MS. Among these patients, there was also a high incidence of constipation and fecal incontinence, which are attributed to demyelination and neurogenic damage in both the central and ENS [91]. Among this cohort, constipation was specifically identified in 82% of study patients, delayed gastric emptying in 16% of patients, and accelerated digastric emptying in 22% of patients [91]. These findings underscore the need for more targeted therapeutics towards addressing enteric dysfunction.
Preziosi and colleagues [68] (2018) also explored the significant impact of bowel dysfunction in MS patients. In this study, constipation was one of the most frequently reported symptoms, characterized by infrequent bowel movements, difficulty in stool passage, and a sensation of incomplete evacuation [68]. Constipation is attributed to many factors including CNS demyelination, ANS dysfunction, and impairments in the ENS [68]. Fecal incontinence and bowel obstruction (often occurring due to severe constipation) are also noted manifestations [68]. Abdominal pain and bloating are other characteristic symptoms, which may be linked to the modulation of autonomic nerve signaling or altered gut motility [68]. Similarly, anorectal dysfunction, which includes difficulties in coordinating the muscles responsible for bowel movements, can contribute to both constipation and incontinence [68].
Emerging evidence suggests that even individuals with newly diagnosed MS and minimal disability may experience severe constipation as an early symptom of the disease [92]. Faber and colleagues [92] (2021) found that neurogenic bowel dysfunction, encompassing constipation, fecal incontinence, and abdominal bloating, ranks among the most burdensome symptoms for MS patients, significantly reducing their quality of life and consuming considerable time in daily management. Supporting this, a survey of 1,334 individuals with spinal cord injuries revealed that 39% experienced constipation, 36% reported hemorrhoids, and 31% suffered from abdominal distension or discomfort, highlighting the widespread and multifactorial nature of GI complications in neurologic disorders [93].
The diagnosis of spinal myelopathy is reached through a combination of clinical assessment, imaging, and functional tests. While the etiologies of myelopathies are myriad, the consequences of a compromised spinal cord present similar signs and symptoms. Thus, a thorough history and physical examination remain fundamental in identifying spinal cord dysfunction. Jiang and colleagues [94] (2024), highlights that the most sensitive indicator of degenerative cervical myelopathy, the most prevalent form of myelopathy, is the presence of hyperreflexia and Tromner signs, whereas Babinski, Tromner, clonus, and inverted supinator signs are considered the most specific. Complementary imaging modalities, particularly MRI, play a critical role in diagnosing myelopathies. Uda and colleagues [95] (2013) measured a level of sensitivity of 100% and a specificity of 75% in diagnosing cervical spine myelopathy using a mean diffusivity (MD) z score of 1.40 at the most compressed level of the spine [95].
To evaluate enteric dysfunction secondary to spinal myelopathy, functional assessments such as high-resolution anorectal manometry, colonic transit studies, and standardized disability indices are employed. High-resolution anorectal manometry measures the rectoanal inhibitory reflex in response to rectal distention, providing valuable insights into conditions like fecal incontinence by assessing anal sphincter strength and rectal sensory function [96, 97]. Colonic transit studies, involving the ingestion of radiopaque markers followed by X-rays taken 3–7 days later, enable the assessment of segmental colon function and transit times. As mentioned earlier, the parasympathetic pathways from S2–S4 play a role in reflexes associated with defecation in the distal portion and can extend the colonic transit time creating a possible proxy [38, 41, 43]. While this method is well-established, the standardization of result interpretation remains under investigation [98]. Alternative methods such as wireless motility assays and capsule endoscopies offer similar functional assessment.
Disability indices, including the myelopathy disability index (MDI) and the modified Japanese Orthopaedic Association (mJOA) score, further quantify the functional impact of myelopathies. The MDI is a questionnaire that describes the difficulty of daily tasks that patients experience while minimizing subjective physician bias. Current advancements in the MDI are establishing severity and then correlating it to the likelihood that surgery will be a benefit [99]. mJOA scores are another popular means of assessing myelopathy induced disability. In this variation, the physician answers questions about the patient’s ability to complete daily activities and retain urine [100]. Additionally, emerging research has been trying to understand the connection between neurotransmitter concentrations, such as cholinergic and serotonergic biomarkers, and clinical parameters like colonic transit time, aiming to elucidate the pathophysiological basis of enteric dysfunction in spinal myelopathies [21].
Management strategies for enteric dysfunction range from invasive procedures, such as the implantation of bioelectric stimulation devices, to pharmacological agents and non-pharmacological treatments like diet-specific interventions. As previously mentioned, the cohorts described by Nouri and colleagues [56] (2020) were more likely to experience GI comorbidities (GIC) when female, with worse general and psychiatric health, along with clinical exams and imaging studies suggesting less neurological complication. This indicates the potential to characterize and specifically target those experiencing GIC more effectively. Given their minimal risk, non-pharmacological treatments should be attempted first. While it is difficult to produce diet related interventions which show causal changes in enteric dysfunction from SCI, Hamilton and colleagues [101] (2024) showed in mice models that inulin, a dietary fiber, and short-chain fatty acids (SCFAs) can attenuate enteric dysfunction by promoting ENS resilience. Other studies have gathered information on the immune and motility related benefits of SCFAs [102]. Exercise has also long been prescribed for chronic constipation, and a meta-analysis by Gao and colleagues [103] (2019) showed that those who exercise by means of walking and other aerobic exercises improved their likelihood of recovery by 2.42 times.
Pharmacological approaches have included, osmotic and stimulatory laxatives, prosecretory agents, and 5HT4 agonists to target constipation secondary to upper motor neuron injury [104]. Osmotic laxatives, such as polyethylene glycol, have been shown to improve stool frequency, consistency, and straining compared to placebo in both functional and irritable bowel syndromes (IBS). Aziz and colleagues [104] (2020) noted that while stimulatory laxatives have less data, agents like bisacodyl and sodium picosulfate have demonstrated superiority to placebo and may be considered if osmotic laxatives fail. Prosecretory agents like linaclotide, plecanatide, and lubiprostone enhance enterocyte biology to increase luminal fluid secretion, providing a cellular mechanism to alleviate constipation (Figure 4). Prucalopride, a 5HT4 agonist, has also shown efficacy in improving constipation and abdominal pain in both functional and opioid-induced constipation [104].
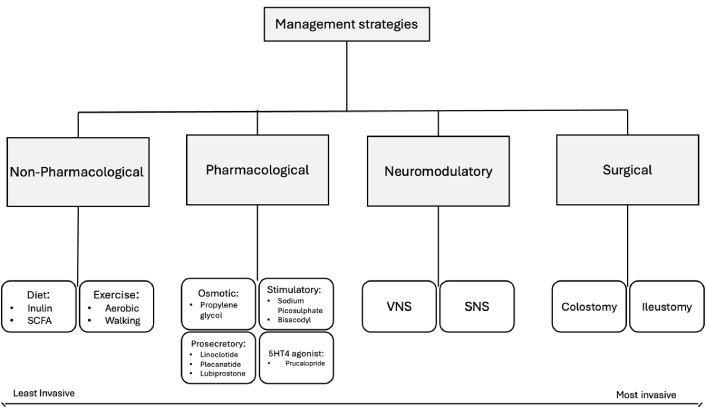
Management strategies for enteric-neuro dysfunction in spinal myelopathy patients. SCFA: short-chain fatty acid; VNS: vagus nerve stimulator; SNS: spinal nerve stimulator
Bioelectric neuromodulation is an intervention that has been gaining traction in multiple pathologies that affect colorectal function including spinal trauma, MS and Hirschsprung disease [105]. By modulating CNS-ENS innervation and increasing lower motor neuron activity, sacral nerve stimulation has demonstrated utility in reducing inflammation in IBS, alleviating postoperative ileus via vagal nerve stimulation, and via sacral nerve stimulation, addressing fecal incontinence via anal sphincter contraction. Such an approach represents an exciting area of development with promising applications for upper motor neuron lesions [105]. As a last resort, colostomy is also an option to mitigate quality-of-life impairments and serves as one of few interventions outside of diet for those with lower motor neuron injuries. With indications for reflexive constipation from upper motor neuron injury and are flexible fecal incontinence from lower motor neuron injury. Both colostomy and ileostomy reduce the amount of time needed to manage bowel and overall, patients have reported a desire to be counseled about this treatment earlier. No significant differences have been observed between colostomy and ileostomy in SCI patients [106].
In order to offset the use of more invasive procedures, sacral and pudenal nerve stimulation (PNS) has emerged as a promising treatment option for patients suffering from intractable constipation [107]. The therapeutic effects of PNS are hypothesized to stem from its interactions with the somatic and autonomic pathways in the spinal cord and higher brain centers, offering a potential solution for neurogenic bowel dysfunction [107]. While this approach holds promise, future research is necessary to provide insight into the long-term efficacy and sustainability of PNS outcomes. In addition, mechanistic research investigating the biological mechanisms behind the effects of the PNS on somatic and autonomic pathways is essential. A deeper understanding of these mechanisms may yield improved treatment protocols, better patient selection, and potentially the development of more targeted therapies that enhance the efficacy of PNS while minimizing adverse side effects.
In patients with neurogenic bowel dysfunction who do not respond to conservative bowel management strategies, transanal irrigation (TAI) has become an emerging therapeutic option. The Navina Smart system, an electronic TAI device, has demonstrated improvement. In a prospective study led by Emmanuel et al. [108], patients reported improvements in bowel function, reduced constipation, decreased incontinence, as well as high satisfaction levels, highlighting Navina Smart’s capabilities as a valuable treatment option. Additionally, patient-centered studies focused on customization and optimization of TAI systems could enhance treatment outcomes by addressing individual patient needs and preferences.
Gut microbiota plays a crucial, yet incompletely understood, role in regulating the inflammatory response and affecting neurological recovery after spinal cord trauma. The gut-brain-spinal axis represents the two-way communication between the gut and CNS [109]. Following an injury, the gut-brain axis contributes to the production of pro-inflammatory metabolites, creating a difficult environment for cell survival and the recovery of movement. Consequently, understanding the relationship between pharmacological treatment and microbiome imbalances is crucial to enhancing regeneration, promoting cell survival, and improving behavioral outcomes [109]. Additionally, proper small bowel motility, governed by intrinsic and extrinsic neurons and humoral factors, is essential for nutrient absorption and digestive health. Impairment in the integrity of coordinated movement of the small bowel, known as small bowel dysmotility, can lead to symptoms such as abdominal pain and distention, constipation, and diarrhea. Given the incomplete understanding of the gut-brain-spinal axis and its complex interactions, further research is necessary.
Mesenchymal stem cells (MSCs) have also gathered attention for their applications in regenerative medicine and their ability to support repair and recovery in spinal cord injuries [110]. Despite their promise, MSC therapies still face significant challenges including optimizing delivery methods, ensuring cell survival, and promoting integration with host tissues [111]. Overcoming these barriers is critical to realizing the full therapeutic potential of MSCs. Continued research and innovation in this field are vital for advancing the treatment of spinal cord injuries and enhancing patient outcomes [111].
Spinal myelopathy-related enteric dysfunction caused by spinal cord compression is commonly associated with dysfunction of spinal cord reflex arcs and the ENS. The disruption of such enteric gut-brain-spinal pathways ultimately contributes to a myriad of clinical presentations and reduced quality of life. Symptoms include gastroparesis, dyspepsia, fecal incontinence, abdominal bloating, constipation, abdominal pain, diarrhea, and chronic neurogenic bowel disease. Enteric dysfunction in the setting of SCI is currently diagnosed and treated with a range of management strategies that encompasses non-pharmacological and pharmacological interventions, neuromodulators, and surgical interventions. However, there remains a lack of complete understanding of ENS dysregulation and its clinical relevance regarding assessment, diagnosis, and treatment options. Future research directions concerning nerve stimulation, treatment options, MSCs, the gut microbiome, and biochemical processes in relation to the gut-brain-spinal nerve axis may prove beneficial in improving clinical outcomes and quality of life for those who suffer from SCI related GI enteric dysregulation.
ANS: autonomic nervous system
CMS: conus medullaris syndrome
CNS: central nervous system
CSM: cervical spondylotic myelopathy
ENS: enteric nervous system
GI: gastrointestinal
MDI: myelopathy disability index
MS: multiple sclerosis
MSCs: mesenchymal stem cells
PNS: pudenal nerve stimulation
SCFAs: short-chain fatty acids
SCI: spinal cord injury
SMAS: superior mesenteric artery syndrome
TAI: transanal irrigation
During the preparation of this work, author(s) used the ChatGPT AI system for improving readability and language of the paper. After using the tool/service, author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
MA: Conceptualization, Project administration, Supervision, Visualization, Writing—original draft, Writing—review & editing. DF: Visualization, Writing—original draft, Writing—review & editing. LB: Visualization, Writing—original draft, Writing—review & editing. BK: Visualization, Writing—original draft, Writing—review & editing. CC: Visualization, Writing—original draft, Writing—review & editing. CR: Visualization, Writing—original draft, Writing—review & editing. BLW: Conceptualization, Project administration, Supervision.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1609
Download: 11
Times Cited: 0
Luxita Sharma, Dhananjay Sharma
Ravi Philip Rajkumar
