Affiliation:
1Department of Botany, Government Brennen College, Dharmadam, Kannur 670106, Kerala, India
Email: deepaabhaskar@gmail.com
ORCID: https://orcid.org/0000-0002-6070-1576
Affiliation:
2Department of Plant Science, Central University of Kerala, Tejaswini Hills, Kasaragod 671325, Kerala, India
Email: den_thuruthiyil@yahoo.com
ORCID: https://orcid.org/0000-0001-9283-5723
Explor Neurosci. 2025;4:100697 DOl: https://doi.org/10.37349/en.2025.100697
Received: February 26, 2025 Accepted: April 27, 2025 Published: June 20, 2025
Academic Editor: Marcello Iriti, Milan State University, Italy
The article belongs to the special issue Medicinal Plants and Bioactive Phytochemicals in Neuroprotection
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and the most common type of dementia, characterized by cognitive decline in later years of life. Among various hypotheses explaining AD pathology, the cholinergic hypothesis is one of the most studied. Though there are Food and Drug Administration (FDA) approved drugs (donepezil, galantamine, rivastigmine and tacrine) for AD treatment, their adverse effects make it urgent to develop new drugs with minimal side effects. This review focuses on the acetylcholinesterase (AChE) inhibitory potential of plant extracts and phytochemicals that could aid in preventing and mitigating AD. From the literature search, extracts of 28 species were found to have strong inhibition against AChE, with IC50 values ranging from 0.08 μg/mL to 10.0 μg/mL. The highest number of species with AChE inhibition belongs to the Amaryllidacea family, followed by Fabaceae, Lycopodiaceae, Amaranthaceae and Anacardiaceae. Several phytochemicals, including alkaloids, terpenoids and phenolics, show a multitarget approach in AD therapy, exhibiting more than one of the following activities such as inhibition of AChE, butyrylcholinesterase (BuChE), MAO-A, beta site amyloid precursor protein cleaving enzyme 1 (BACE-1), β-amyloid (Aβ) aggregation, tau phosphorylation, and an ability to cross blood-brain barrier (BBB). With a multitarget approach and minimal side effects, they could revolutionise the treatment of AD. Many phytochemicals and their derivatives are under clinical and pre-clinical trials, potentially serving as prospective therapeutic drug candidates for treating AD. This review briefly discusses the findings and advances in knowledge about plant-derived bioactive compounds as potential new drugs acting as AChE inhibitors.
Alzheimer’s disease (AD) is the most predominant type of dementia, accounting for approximately 60–80% of all cases worldwide [1–3]. Around 50 million people across the globe were living with AD as of 2020, and a twofold increase is anticipated every 20 years, exceeding 90 million by 2050 [1]. This progressive and irreversible neurodegenerative disorder primarily affects individuals over the age of 60, with a global prevalence of 4.02% and an annual incidence rate of 3.41% [2, 4]. As per the current statistics, during the COVID-19 pandemic, death cases have escalated by 16 per cent [5]. The symptoms of AD are manifested only after years of onset of the disease [6]. Early symptoms include apathy, inability to recall recent incidents or names and depression. As the condition progresses, it leads to disorientation, communication issues, poor judgement, confusion and behavioural changes. The late-stage symptoms include difficulty speaking, walking and swallowing [5].
Though the pathology of AD is not entirely acknowledged, several factors contributing to neurodegeneration have been identified so far [7–10]. One of the major pathological features of AD is the accumulation of beta-amyloid plaques in the brain, leading to nerve loss, especially in the hippocampus region, which is the site of memory, learning and emotions [11]. Increased levels of hyperphosphorylated tau proteins associated with neurofibrillary tangles are other pathological hallmarks of AD [12–14]. Patients with AD have shown reduced levels of acetylcholine (Ach) in the synapses [15]. It was not until recently that the dual roles of acetylcholinesterase (AChE), including the formation of beta-amyloid plaques in brain cells and the hydrolysis of neurotransmitters, were unveiled [11]. These diverse roles make AChE a potential target for AD treatment [11].
ACh is a neurotransmitter crucial in learning, memory and synaptic plasticity. A key indicator of AD is the depletion of ACh [15]. AChE is a key enzyme which plays a vital role in regulating nerve impulse transmission at cholinergic synapses by catalyzing the breakdown of ACh [16]. Normal neurotransmission occurs when ACh is released into the synaptic cleft from the presynaptic neuron, which then binds to the ACh receptors on the postsynaptic membrane. This initiates a relay of signals from the nerve. AChE is also present in the postsynaptic membrane and catalyzes the hydrolysis of ACh into choline and acetate to terminate the signal. The choline then re-enters the presynaptic neuron where choline-acetyl transferase combines it with acetyl-CoA to rebuild ACh [17]. In AD, the hyperactivity of AChE aggravates the breakdown of ACh, thereby disrupting cholinergic signalling and leading to cognitive decline [18]. Recent findings have highlighted the influence of AChE in beta-amyloid plaque formation, suggesting its role in the advancement of AD. This bifunctionality of AChE as a hydrolytic enzyme and promoter of amyloid aggregation makes it a crucial target in the therapeutic management of AD [19]. Studies highlight the long-term health benefits of AChE inhibitors as they are associated with slower cognitive decline and reduced mortality in older adults with dementia [20]. Recent studies have identified novel heterocyclic compounds with AChE inhibitory properties, such as coumarin-benzotriazole hybrids and carbazole derivatives. They act on both the catalytic active site and peripheral anionic site (PAS) of AChE, along with low toxicity profiles and high blood-brain barrier (BBB) permeability, offering potential leads for AD treatment [21].
Current treatment of AD is mainly based on four licensed medications that are acetylcholinesterase inhibitors (AChEIs). These include donepezil, galantamine, rivastigmine and tacrine, which prevent the breakdown of ACh and increase impulse transmission at cholinergic sites, with rivastigmine exhibiting the highest effect [15, 22, 23]. Unfortunately, these drugs have minimal bioavailability and they only offer symptomatic relief and temporary improvement in cognitive function, failing to stop or invert neurodegeneration [24]. On top of that, the continuous use of these drugs causes multiple side effects such as nausea, vomiting and gastrointestinal disorders, restricting their prolonged use [14]. Here comes the need to identify safer and more effective AChEIs targeting multiple aspects of AD pathology.
A great deal of research has been conducted in the last decade on plant-derived bioactive compounds with AChEI potential. Several plants with promising AChEI activity have been used in traditional medical systems, such as Ayurveda and traditional Chinese medicine, for their neuroprotective and memory-boosting potential [1, 25]. These plants contain various phytochemicals, including alkaloids, flavonoids, terpenoids, and many other phenolic compounds with antioxidant, anti-amyloidogenic and anti-inflammatory properties in addition to demonstrated AChEI activity [15, 26]. Studies show that plants of Amaranthaceae, Amaryllidaceae, Lycopodiaceae, Myristicaceae, Polygonaceae, Rutaceae and Valerianaceae are potential sources of AChEIs [1, 11]. This review focuses on recent research related to plant-based AChEIs and summarises the potential plants and phytochemicals with demonstrated AChEI activity.
The current Food and Drug Administration (FDA) approved drugs for the treatment of AD include cholinesterase inhibitors such as donepezil, rivastigmine, galantamine and glutamate receptor antagonist memantine. These inhibitors bind to the enzyme and prevent it from hydrolyzing Ach, activating cholinergic signalling and reducing the symptoms of AD [27]. Tacrine was the first cholinesterase inhibitor drug approved by the FDA for the symptomatic relief of AD, but it is no longer in use as it causes hepatotoxicity [28]. Though these second-generation cholinesterase inhibitors are classified under the same class of medication, they belong to different chemical classes and are distinct in their pharmacological properties [29].
Rivastigmine is a phenyl-carbamate derivative (Figure 1), frequently referred to as a pseudo-irreversible cholinesterase inhibitor, as its plasma effects persist longer than expected. It is the only drug that inhibits both AChE and butyrylcholinesterase (BuChE) and it is available in both oral capsules and transdermal patches [29]. Upon absorption, it acts like ACh by binding both the anionic and esteratic sites of AChE [30]. Unlike ACh, which dissociates immediately after hydrolysis, rivastigmine gets hydrolyzed by leaving the esteratic site of AChE carbamylated for a while, inhibiting the enzyme [30]. FDA has approved a rivastigmine dosage of 4.6 mg/day of oral capsule and 9.5 mg/day of transdermal patch for mild-moderate stages of AD and 13.3 mg/day for severe stages [31, 32]. The availability of a carrier protein in the blood dramatically influences the performance of an administered drug. Being closely related to human serum albumin, bovine serum albumin is often used in conjugation with rivastigmine tartrate and has shown promising results in treating AD [33]. Rivastigmine has also exhibited a potential role in directing the amyloid precursor protein into a non-amyloidogenic pathway [34]. These benefits of rivastigmine are also associated with adverse effects. While the most frequent side effects are gastrointestinal problems such as nausea, vomiting, diarrhoea, abdominal pain, dizziness, headache, anorexia and fatigue, confusion and agitation are less common. Skin reactions are the next most common side effect seen only in transdermal patches. Though the side effects are non-lethal, an overdose of the drug in any form can be fatal [29].
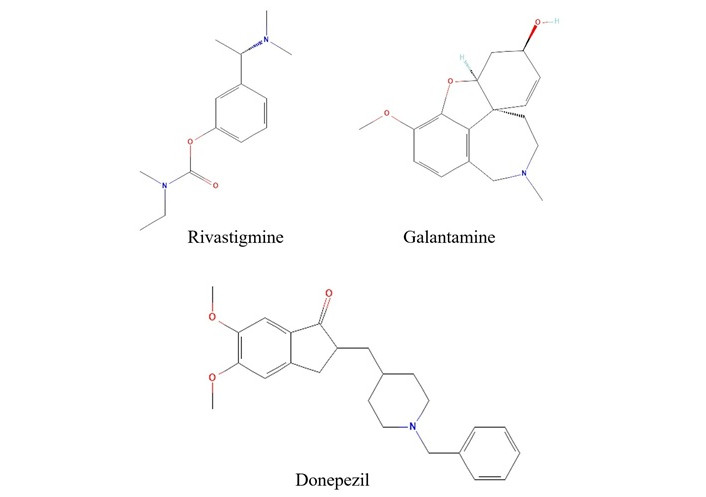
Structure of current FDA-approved anti-cholinesterase drugs.
Note. Data retrieved from PubChem. Rivastigmine: PubChem Identifier: CID 77991; URL: https://pubchem.ncbi.nlm.nih.gov/compound/77991#section=2D-Structure; Galantamine: PubChem Identifier: CID 9651; URL: https://pubchem.ncbi.nlm.nih.gov/compound/9651#section=2D-Structure; Donepezil: PubChem Identifier: CID 3152; URL: https://pubchem.ncbi.nlm.nih.gov/compound/3152#section=2D-Structure
Donepezil is a piperidine-based second-generation inhibitor (Figure 1) of AChE. It consists of a benzylpiperidine moiety linked to dimethoxy indanone by the methylene group [35]. It is a selective, reversible, and non-competitive inhibitor of AChE that binds to its anionic site [36, 37]. Even though a cholinesterase inhibitor, donepezil has a more inhibitory effect on AChE than BuChE [37–39]. In addition, an increased absorption time (3–5 hours) and a long half-life of 70 hours reduce the frequency of tablet consumption. Due to its affinity to serum albumin, donepezil is easily excreted through urine [37, 39]. In vitro studies have shown the potential of deoxyvasicinone-donepezil hybrids as a multitarget drug in alleviating AD [40]. Donepezil-loaded nano drugs have displayed a dual role in inhibiting AChE and β-amyloid (Aβ)-targeting clearance [41, 42]. Despite being an AChEI used in the treatment of AD, donepezil has been reported to have several adverse effects due to elevated levels of ACh beyond the central nervous system. The side effects include cardiovascular problems such as bradycardia, QTc prolongation and a life-threatening arrhythmia, Torsades de Pointes [43]. Studies have also shown that donepezil alters sleep architecture [44], and an increase in daily dosage has induced parkinsonism [45]. Other adverse effects include nausea, vomiting, diarrhoea, anorexia, muscle cramps, fatigue, insomnia and increased gastrointestinal secretions [37, 39, 46, 47].
Galantamine is a heterocyclic phenanthridine derivative (Figure 1) isolated from the flowers and bulbs of Galanthus woronowi of the Amaryllidaceae family. It is the only FDA-approved alkaloid drug used for the treatment of AD [48]. Despite its relatively limited inhibitory activity, it serves efficiently as an allosteric modulator of the nicotinic ACh receptor. The selective inhibition of AChE in the central nervous system with minimal effect on the peripheral nervous system makes galantamine a preferred candidate for AD treatment. Early-stage dosage of galantamine has notably reduced oxidative stress and lowered the production of proinflammatory cytokines, resulting in enhanced cognitive function [49]. Recent studies focus on the synergistic effect of compounds. A conjugate of galantamine and memantine has shown neuroprotectivity in a dual path, inhibiting AChE and alleviating N-methyl-D-aspartate induced neurotoxicity [50]. Transdermal delivery is a more recent approach for administering galantamine-memantine conjugate, which was found effective in bypassing the hepatic first-pass metabolism [51]. Regardless of its AChEI potential, galantamine, like all other AChEIs, has several adverse effects, including gastrointestinal problems such as nausea, vomiting, diarrhoea and anorexia, which may increase with dosage [52]. Other side effects of galantamine administration include urinary retention, QT prolongation, sinus bradycardia, syncope, and delirium [52].
The reports suggest that rivastigmine is a dual inhibitor of AChE and BuChE, while galantamine and donepezil are highly selective for AChE. On comparing the efficiency and side effects, the rivastigmine transdermal patch has the upper hand, showing mild side effects compared to others, especially with gastrointestinal problems. However, the best choice always depends on the patient’s other underlying conditions. Although these are FDA-approved drugs, they still have side effects that are dose-limiting, such as gastrointestinal problems, cardiovascular complications, insomnia, anorexia, fatigue, and confusion [29, 52]. There comes the significance of safer and multifunctional plant-based drugs. There is an array of plant-derived bioactive compounds that are multifunctional and have neuroprotective, antioxidant, and anti-inflammatory properties. Reports suggest that phytochemicals such as alkaloids and terpenoids regulate multiple pathological pathways of AD, including tau protein phosphorylation, beta-amyloid plaque formation, and oxidative stress, in addition to AChE inhibition [53]. Many phytochemicals are also involved in the inhibition of beta-site amyloid precursor protein cleaving enzyme 1 (BACE-1) and monoamine oxidase (MAO) [54, 55]. This suggests the potential of phytochemicals in offering a pathology-based treatment for AD rather than symptomatic relief.
Since time immemorial, medicinal plants have been identified, cultivated, and used in traditional medicines. Traditional medicinal systems such as Ayurveda, Unani, Siddha, and Chinese traditional medicine all employ medicinal plants and extracts to treat numerous illnesses. According to the World Health Organization (WHO), around 80% of people across the world use herbal medicines in primary health care [56]. This points to the presence of pharmacological lead molecules in medicinal plants and their therapeutic effects. Herbal remedies are employed worldwide to treat neurological conditions, including those caused by impaired AChE activity [56]. Research has revealed the AChEI of numerous plant extracts (Table 1).
Plant extracts with AChEI activity and their IC50 value
| Plant | Family | Plant part | Type of fraction/extract | IC50(μg/mL) | Positive control(IC50) | References |
|---|---|---|---|---|---|---|
| Citharexylum spinosum L. | Verbenaceae | Leaf | Hexane-benzene fraction | 0.08 | Donepezil (0.05 μg/mL) | [57] |
| Myrciaria floribunda | Myrtaceae | Fruit peel | Essential oil | 0.08 | Neostigmine (23.3 μg/mL) | [58] |
| Carpolobia lutea G. Don | Polygalaceae | Root | Ethyl acetate fraction | 0.3 | Physostigmine (0.2 μg/mL) | [59] |
| Lannea schweinfurthii Engl. | Anacardiaceae | Root | Ethyl acetate extract | 0.3 | Galantamine (0.05 μg/mL) | [60] |
| Scadoxus puniceus (L.) Friis & Nordal | Anacardiaceae | Bulb | Ethyl acetate extract | 0.3 | Galantamine (0.05 μg/mL) | [60] |
| Xysmalobium undulatum (L.) W. T. Aiton | Apocynaceae | Root | Ethyl acetate extract | 0.5 | Galantamine (0.05 μg/mL) | [60] |
| Citharexylum spinosum L. | Verbenaceae | Leaf | n-Butanol fraction | 0.6 | Donepezil (0.05 μg/mL) | [57] |
| Huperzia tetragona (Hook. & Grev.) Trevis | Lycopodiaceae | Aerial parts | Alkaloidal fraction | 0.9 | Donepezil (0.036 μg/mL) | [61] |
| Esenbeckia leiocarpa Engl. | Rutaceae | Stem | Alkaloidal fraction | 1.6 | Galantamin (1.7 µM) | [62] |
| Citharexylum spinosum L. | Verbenaceae | Leaf | Hydro-alcohol (50%) fraction | 1.86 | Donepezil (0.05 μg/mL) | [57] |
| Rumex acetosa L. | Polygonaceae | Leaf | Hydro alcohol extract | 1.93 | Not mentioned | [63] |
| Carpolobia lutea G. Don | Polygalaceae | Root | Aqueous fraction | 2.0 | Physostigmine (0.2 μg/mL) | [59] |
| Crinum Bulbispermum (Burm. f.) Milne-Redh. & Schweick. | Amaryllidaceae | Bulb | Ethyl acetate extract | 2.1 | Galantamine (0.05 μg/mL) | [60] |
| Citharexylum spinosum L. | Verbenaceae | Leaf | Chloroform fraction | 2.26 | Donepezil (0.05 μg/mL) | [57] |
| Morus alba L. | Moraceae | Root-bark | Ethyl acetate fraction | 2.5 | Berberine (0.13 mg/mL) | [64] |
| Angelica decursiva (Miq.) Franch. & Sav. | Apiaceae | Whole plant | Aqueous fraction | 2.6 | Berberine (0.07 μg/mL) | [65] |
| Crinum moorei Hook.f. | Amaryllidaceae | Bulb | Dichloromethane fraction | 2.9 | Galantamine (0.33 μg/mL) | [66] |
| Geissospermum laeve (Vell.) Miers | Apocynaceae | Stem bark | Indole Alkaloids | 2.9 | Physostigmine (6.61 μg/mL) | [67] |
| Carpolobia lutea G. Don | Polygalaceae | Root | Methanolic extract | 3.0 | Physostigmine (0.2 μg/mL) | [59] |
| Citharexylum spinosum L. | Verbenaceae | Leaf | Hexane fraction | 3.57 | Donepezil (0.05 μg/mL) | [57] |
| Taraxacum officinale L. Weber ex F.H. Wigg | Asteraceae | Leaf | Hydro alcohol extract | 4.27 | Not mentioned | [63] |
| Hypericum perforatum L. | Hypericaceae | Aerial parts | Hydro alcohol extract | 4.32 | Not mentioned | [63] |
| Citharexylum spinosum L. | Verbenaceae | Leaf | Ethyl acetate fraction | 4.8 | Donepezil (0.05 μg/mL) | [57] |
| Buchanania axillaris (Desr.) Ramamoorthy | Anacardiaceae | Aerial parts | 90% methanol fraction | 4.96 | Galantamine (0.77 μg/mL) | [68] |
| Salvia miltiorrhiza Bunge | Lamiaceae | Root | Ethanolic extract | 5.0 | Not mentioned | [69] |
| Huperzia serrata | Lycopodiaceae | Aerial parts | Alkaloidal fraction | 5.96 | Donepezil (0.02 μg/mL) | [70] |
| Rauvolfia serpentina (L.) Bth.ex Kurz | Apocyanaceae | Root | n-Butanol fraction | 5.99 | Galantamine (0.63 μg/mL) | [71] |
| Angelica decursiva (Miq.) Franch. & Sav. | Apiaceae | Whole plant | Buthanolic fraction | 6.0 | Berberine (0.07 μg/mL) | [65] |
| Esenbeckia leiocarpa Engl. | Rutaceae | Stem | Hexanic fraction | 6.0 | Galantamin (1.7 µM) | [62] |
| Horsfieldia tomentosa (Warb) | Myristicaceae | Fruit | Ethyl acetate extract | 6 | Eserine (27.53 μg/mL) | [72] |
| Byrsonima sericea DC. | Malpighiaceae | Pulp/Peel | Ethanol extract | 6.02 | Galantamine (1.02 μg/mL) | [73] |
| Colocasia antiquorum Schott | Araceae | Tubers | Petroleum ether fraction | 6.4 | Galantamine (0.33 μg/mL) | [66] |
| Byrsonima sericea DC. | Malpighiaceae | Seed | Ethanol extract | 6.64 | Galantamine (1.02 μg/mL) | [73] |
| Rauvolfia serpentina (L.) Bth.ex Kurz | Apocyanaceae | Root | Ethanol extract | 7.46 | Galantamine (0.63 μg/mL) | [71] |
| Berberis aetnensis C. Presl | Berberidaceae | Root | Methanolic fraction | 7.6 | Physostigmine (0.00017) | [74] |
| Colocasia antiquorum Schott | Araceae | Tubers | 50% methanol fraction | 7.9 | Galantamine (0.33 μg/mL) | [66] |
| Paeonia lactiflora Pall. | Ranunculaceae | Root | Ethanolic extract | 8.0 | Not mentioned | [69] |
| Pycnostachys reticulata (E.Mey.) Benth. | Lamiaceae | Root | Ethanol extract | 8.8 | Galantamine (0.33 μg/mL) | [66] |
| Marrubium vulgare L. | Lamiaceae | Leaf | Hydro alcohol extract | 8.92 | Not mentioned | [63] |
| Spatholobus suberectus Dunn. | Leguminosae | Stem | Ethanolic extract | 9.0 | Not mentioned | [69] |
| Byrsonima sericea DC. | Malpighiaceae | Seed | Hexane extract | 9.04 | Galantamine (1.02 μg/mL) | [73] |
| Horsfieldia tomentosa (Warb) | Myristicaceae | Fruit | n-Hexane non-soluble fraction | 9.1 | Eserine (27.53 μg/mL) | [72] |
| Senna obtusifolia (L.) H. S. Irwin & Barneby | Leguminosae | Leaf | Ethyl acetate fraction | 9.4 | Berberine (0.07 mg/mL) | [75] |
| Angelica decursiva (Miq.) Franch. & Sav. | Apiaceae | Whole plant | Ethyl acetate fraction | 9.7 | Berberine (0.07 μg/mL) | [65] |
| Senna obtusifolia (L.) H. S. Irwin & Barneby | Leguminosae | Leaf | Buthanolic fraction | 9.9 | Berberine (0.07 mg/mL) | [75] |
| Zanthoxylum davyi Waterm. | Rutaceae | Root | Methanolic extract | 10 | Galantamine (0.05 μg/mL) | [60] |
| Uncaria rhynchophylla (Miq.) Miq. ex Havil. | Rubiaceae | Stem | Total alkaloids | 10.8 | Huperzine A (0.074 μg/mL) | [76] |
| Byrsonima sericea DC. | Malpighiaceae | Pulp/Peel | Hexane extract | 10.92 | Galantamine (1.02 μg/mL) | [73] |
| Hypericum heterophyllum Vent. | Hypericaceae | Leaf | Acetone extract | 11.07 | Not mentioned | [77] |
| Ziziphus mucronata Willd. | Rhamnaceae | Root | Ethyl acetate extract | 11.2 | Galantamine (0.05 μg/mL) | [60] |
| Morus alba L. | Moraceae | Root-bark | Methanolic extract | 11.4 | Berberine (0.13 mg/mL) | [64] |
| Zanthoxylum davyi Waterm. | Rutaceae | Root | Ethyl acetate extract | 11.6 | Galantamine (0.05 μg/mL) | [60] |
| Nelumbo nucifera Gaertn. | Nelumbonaceae | Leaf | Total alkaloids | 12.2 | Huperzine A (0.074 μg/mL) | [78] |
| Buchanania axillaris (Desr.) Ramamoorthy | Anacardiaceae | Aerial parts | Chloroform fraction | 12.29 | Galantamine (0.77 μg/mL) | [68] |
| Hypericum heterophyllum Vent. | Hypericaceae | Leaf | Chloroform extract | 12.33 | Not mentioned | [77] |
| Senna obtusifolia (L.) H. S. Irwin & Barneby | Leguminosae | Leaf | Chloroform fraction | 12.7 | Berberine (0.07 mg/mL) | [75] |
| Polygonum multiflorum Thunb | Polygonaceae | Root | Aqueous extract | 13.0 | Not mentioned | [69] |
| Centauricum erythrea Rafn. | Gentianaceae | Aerial parts | Hydro alcohol extract | 13.11 | Not mentioned | [63] |
| Morus alba L. | Moraceae | Root-bark | Chloroform fraction | 13.4 | Berberine (0.13 mg/mL) | [64] |
| Horsfieldia tomentosa (Warb) | Myristicaceae | Fruit | Methanol extract | 13.5 | Eserine (27.53 μg/mL) | [72] |
| Angelica decursiva (Miq.) Franch. & Sav. | Apiaceae | Whole plant | Chloroform fraction | 13.7 | Berberine (0.07 μg/mL) | [65] |
| Ceratonia siliqua L. | Fabaceae | Fruit | Hydro alcohol extract | 13.9 | Not mentioned | [63] |
| Hypericum heterophyllum Vent. | Hypericaceae | Leaf | Ethanol extract | 13.99 | Not mentioned | [77] |
| Senna obtusifolia (L.) H. S. Irwin & Barneby | Leguminosae | Leaf | Aqueous fraction | 14.5 | Berberine (0.07 mg/mL) | [75] |
| Crinum Bulbispermum (Burm. f.) Milne-Redh. & Schweick. | Amaryllidacea | Bulb | Methanolic extract | 14.8 | Galantamine (0.05 μg/mL) | [60] |
| Scabiosa arenaria Forssk. | Caprifoliaceae | Stem and Leaf | Ethyl acetate fraction | 16 | Eserine (0.003 μg/mL) | [79] |
| Hypericum heterophyllum Vent. | Hypericaceae | Leaf | Methanol extract | 16.32 | Not mentioned | [77] |
| Angelica decursiva (Miq.) Franch. & Sav. | Apiaceae | Whole plant | Methanolic extract | 16.6 | Berberine (0.07 μg/mL) | [65] |
| Berberis vulgaris L. | Berberidaceae | Fruit | Hydro alcohol extract | 16.8 | Not mentioned | [63] |
| Glycyrrhiza glabra L. | Fabaceae | Stem | Hydro alcohol extract | 16.8 | Not mentioned | [63] |
| Berberis libanotica Ehrenb. ex C.K. Schneid. | Berberidaceae | Root | Methanolic fraction | 16.9 | Physostigmine (0.00017) | [74] |
| Zanthoxylum nitidum (Roxb.) DC. | Rutaceae | Root | Total alkaloids | 17.4 | Huperzine A (0.074 μg/mL) | [76] |
| Pavetta indica L. | Rubiaceae | Aerial parts | Methanolic extract | 17.8 | Galantamine (0.77 μg/mL) | [80] |
| Zephyranthes carinata Herb. | Amaryllidaceae | Bulb | Alkaloidal fraction | 18.0 | Galantamine (1.55 μg/mL) | [81] |
| Hippeastrum hybridum | Amaryllidaceae | Whole plant | 70 % methanol extract | 18.1 | Not mentioned | [82] |
| Crinum jagus (J. Thomps.) Dandy | Amaryllidaceae | Bulb | Alkaloidal fraction | 18.3 | Galantamine (1.55 μg/mL) | [81] |
| Annona Squamosa L. | Annonaceae | Pulp | Methanol extract | 18.82 | Physostigmine (1.15 μg/mL) | [83] |
| Adenia gummifera (Harv.) Harms | Passifloraceae | Root | Ethyl acetate extract | 18.9 | Galantamine (0.05 μg/mL) | [60] |
| Crinum moorei Hook.f. | Amaryllidaceae | Bulb | Petroleum ether extract | 18.9 | Galantamine (0.33 μg/mL) | [66] |
| Crataegus monogyna Jacq. | Rosaceae | Fruit | Hydro alcohol extract | 19 | Not mentioned | [63] |
| Harpephyllum caffrum Bernh. ex Krauss | Anacardiaceae | Stem bark | Methanol extract | 20 | Galantamine (0.37 μg/mL) | [84] |
| Paeonia lactiflora Pall. | Ranunculaceae | Root | Aqueous extract | 20.0 | Not mentioned | [69] |
| Rumex hastatus D. Don | Polygonaceae | Whole plant | Flavonoid fraction | 20.0 | Galantamine (20.0 μg/mL) | [85] |
| Horsfieldia tomentosa (Warb) | Myristicaceae | Fruit | n-Hexane soluble fraction | 21.2 | Eserine (27.53 μg/mL) | [72] |
| Crinum moorei Hook.f. | Amaryllidaceae | Bulb | 50% methanol extract | 21.5 | Galantamine (0.33 μg/mL) | [66] |
| Berberis libanotica Ehrenb. ex C.K. Schneid. | Berberidaceae | Root | Methanolic extract | 21.7 | Physostigmine (0.00017) | [74] |
| Searsia mysorensis (G. Don) Moffett. | Anacardiaceae | Aerial parts | 90% methanol | 21.73 | Galantamine (0.77 μg/mL) | [68] |
| Annona Squamosa L. | Annonaceae | Seed | Methanol extract | 22.31 | Physostigmine (1.15 μg/mL) | [83] |
| Crinum moorei Hook.f. | Amaryllidaceae | Bulb | Ethanol extract | 22.5 | Galantamine (0.33 μg/mL) | [66] |
| Huperzia squarrosa (G. Forst.) Trevis. | Lycopodiaceae | Aerial parts | Ethyl acetate fraction | 23.4 | Berberine chloride (0.28 μg/mL) | [86] |
| Rauvolfia serpentina (L.) Bth.ex Kurz | Apocyanaceae | Root | Ethyl acetate fraction | 23.62 | Galantamine (0.63 μg/mL) | [71] |
| Berberis aetnensis C. Presl | Berberidaceae | Root | Alkaloidal fraction | 24.5 | Physostigmine (0.00017) | [74] |
| Cissampelos sympodialis Eichler | Menispermaceae | Root | Alkaloidal fraction | 25.19 | Physostigmine (1.15 μg/mL) | [87] |
| Ochna obtusata DC. | Ochnaceae | Aerial parts | Chloroform fraction | 25.7 | Galantamine (0.77 μg/mL) | [80] |
| Fraxinus excelsior L. | Oleaceae | Fruit | Hydro alcohol extract | 26 | Not mentioned | [63] |
| Gossypium herbaceum L. | Malvaceae | Flower | Hydroalcoholic extracts | 28.1 | Huperzine A (0.01 μg/mL) | [88] |
| Hippeastrum barbatum Herb | Amaryllidaceae | Bulb | Alkaloid fraction | 28.1 | Galantamine (1.55 μg/mL) | [81] |
| Hemidesmus indicus (L.) R. Br. ex Schult. | Apocynaceae | Aerial parts | Chloroform fraction | 28.14 | Galantamine (0.77 μg/mL) | [68] |
| Pycnostachys reticulata (E.Mey.) Benth. | Lamiaceae | Root | 50% methanol extract | 28.8 | Galantamine (0.33 μg/mL) | [66] |
| Scabiosa arenaria Forssk. | Caprifoliaceae | Stem and Leaf | Buthanolic fraction | 29.0 | Eserine (0.003 μg/mL) | [79] |
| Sarcocephalus latifolius (sm.) | Rubiaceae | Fruit | Fatty acid methyl esters | 29.14 | Donepenzil (127.70 μg/mL) | [89] |
| Senna obtusifolia (L.) H. S. Irwin & Barneby | Leguminosae | Leaf | Methanolic fraction | 29.2 | Berberine (0.07 mg/mL) | [75] |
| Portulaca oleracea L. | Portulacaceae | Upper part | Total alkaloids | 29.4 | Huperzine A (0.074 μg/mL) | [76] |
| Salsola kali subsp. tragus (L.) Cˇelak. | Amaranthaceae | Aerial parts | Tetrahydroisoquinoline alkaloids | 30.2 | Physostigmine (0.2 μg/mL) | [90] |
| Ficus sur Forssk. | Moraceae | Fruit | Ethyl acetate extract | 31.9 | Galantamine (0.05 μg/mL) | [60] |
| Rumex hastatus D. Don | Polygonaceae | Aerial parts | Essential oils | 32.5 | Galantamine (4.73 μg/mL) | [91] |
| Rumex hastatus D. Don | Polygonaceae | Aerial parts | Essential oils | 32.5 | Galantamine (20.0 μg/mL) | [91] |
| Acalypha alnifolia Klein ex Willd. | Euphorbiaceae | Aerial parts | Chloroform fraction | 32.9 | Galantamine (0.77 μg/mL) | [80] |
| Nelumbo nucifera Gaertn. | Nelumbonaceae | Embryo | Buthanolic fraction | 33.2 | Eserine (0.02 μg/mL) | [92] |
| Olax nana Wall. ex Benth. | Olacaceae | Leaf | Methanolic extract | 33.2 | Galantamine (19.26 μg/mL) | [93] |
| Persicaria hydropiper (L.) Delarbre. | Polygonaceae | Whole plant | Hexanic fraction | 35.0 | Galantamine (0.1 μg/mL) | [94] |
| Zizipus lotus (L.) Lam | Rhamnaceae | Fruit | Hydro alcohol extract | 35 | Not mentioned | [63] |
| Berberis aetnensis C. Presl | Berberidaceae | Root | Hexanic fraction | 36.5 | Physostigmine (0.00017) | [74] |
| Morus alba L. | Moraceae | Root-bark | Buthanolic fraction | 36.6 | Berberine (0.13 mg/mL) | [64] |
| Petiveria alliaceae L. | Phytolaccaceae | Leaf | Methanol extract | 36.6 | Eserine (0.11 μg/mL) | [95] |
| Aloe ferox Mill. | Xanthorrhoeaceae | Leaf | Petroleum ether fraction | 37.7 | Galantamine (0.33 μg/mL) | [66] |
| Zephyranthes minuta (Kunth) D.Dietr. | Amaryllidaceae | Bulb | Amaryllidaceae alkaloids | 39.2 | Galantamine (6.9 μg/mL) | [96] |
| Crinum Bulbispermum (Burm. f.) Milne-Redh. & Schweick. | Amaryllidaceae | Root | Ethyl acetate extract | 39.3 | Galantamine (0.05 μg/mL) | [60] |
| Huperzia brevifolia (Grev. & Hook.) Holub | Lycopodiaceae | Aerial parts | Alkaloidal fraction | 39.6 | Donepezil (0.036 μg/mL) | [61] |
| Illicium verum Hook.f. | Schisandraceae | Fruit | Essential oil | 39.89 | Galantamine (10.14 μg/mL) | [97] |
| Wedelia chinensis | Asteraceae | Whole | aqueous fraction | 40.02 | Donepenzil (9.21 μg/mL) | [98] |
| Piper capense L. f. | Piperaceae | Root | Ethyl acetate extract | 40.7 | Galantamine (0.05 μg/mL) | [60] |
| Searsia mysorensis (G. Don) Moffett. | Anacardiaceae | Aerial parts | Chloroform fraction | 41.35 | Galantamine (0.77 μg/mL) | [68] |
| Morus alba L. | Moraceae | Root-bark | Aqueous fraction | 43.0 | Berberine (0.13 mg/mL) | [64] |
| Nonea micrantha Bioss. & Reut | Boraginaceae | Whole | Hexane fraction | 44 | Galantamine (0.1 μg/mL) | [99] |
| Illicium verum Hook.f. | Schisandraceae | Fruit | Butanol fraction | 44.94 | Galantamine (10.14 μg/mL) | [97] |
| Elaeagnus umbellata Thunb | Elaeagnaceae | Fruit | Essential oil | 48 | Galantamine (25 μg/mL) | [100] |
| Hemidesmus indicus (L.) R. Br. ex Schult. | Apocynaceae | Aerial parts | 90% methanol extract | 48.64 | Galantamine (0.77 μg/mL) | [68] |
| Jatropha gossypifolia L. | Euphorbiaceae | Leaf | Methanol extract | 50 | Galantamine (0.37 μg/mL) | [101] |
| Salvia miltiorrhiza Bunge | Lamiaceae | Root | Aqueous extract | 50.0 | Not mentioned | [69] |
| Huperzia squarrosa (G. Forst.) Trevis. | Lycopodiaceae | Aerial parts | Buthanolic fraction | 50.1 | Berberine chloride (0.28 μg/mL) | [86] |
| Esenbeckia leiocarpa Engl. | Rutaceae | Stem | Ethanolic extract | 50.7 | Galantamin (1.7 µM) | [62] |
| Scabiosa arenaria Forssk. | Caprifoliaceae | Flower | Ethyl acetate fraction | 51.0 | Eserine (0.003 μg/mL) | [79] |
| Pinus heldreichii Christ | Pinaceae | Needle | Essential oil | 51.1 | Galantamine (0.3 μg/mL) | [102] |
| Pavetta indica L. | Rubiaceae | Aerial parts | Chloroform fraction | 52.1 | Galantamine (0.77 μg/mL) | [80] |
| Persicaria hydropiper (L.) Delarbre. | Polygonaceae | Whole plant | Chloroform fraction | 55.0 | Galantamine (0.1 μg/mL) | [94] |
| Pinellia ternata (Thunb.) Makino | Araceae | Tuber | Total alkaloids | 56.2 | Huperzine A (0.074 μg/mL) | [76] |
| Wedelia chinensis | Asteraceae | Whole | Ethylacetate fraction | 57.76 | Donepenzil (9.21 μg/mL) | [98] |
| Illicium verum Hook.f. | Schisandraceae | Fruit | Hydro-alcoholic extract | 58.67 | Galantamine (10.14 μg/mL) | [97] |
| Acalypha alnifolia Klein ex Willd. | Euphorbiaceae | Aerial parts | Methanolic extract | 59.2 | Galantamine (0.77 μg/mL) | [80] |
| Carpolobia lutea G. Don | Polygalaceae | Leaf | Chloroform fraction | 60 | Physostigmine (0.2 μg/mL) | [59] |
| Pavetta indica L. | Rubiaceae | Aerial parts | Buthanolic fraction | 60.1 | Galantamine (0.77 μg/mL) | [80] |
| Nelumbo nucifera Gaertn. | Nelumbonaceae | Embryo | Ethylacetate fraction | 61.1 | Eserine (0.02 μg/mL) | [92] |
| Sanguinaria candensis L. | Papaveraceae | Whole plant (during flowering) | Ethyl acetate extract | 61.24 | Galantamine (0.42 μg/mL) | [103] |
| Huperzia compacta (Hook.) Trevis. | Lycopodiaceae | Aerial parts | Alkaloid fraction | 62.4 | Donepezil (0.036 μg/mL) | [61] |
| Aloe ferox Mill. | Xanthorrhoeaceae | Leaf | Dichloromethane fraction | 62.6 | Galantamine (0.33 μg/mL) | [66] |
| Salsola soda L. | Amaranthaceae | Aerial parts | Tetrahydroisoquinoline alkaloids | 64.1 | Physostigmine (0.2 μg/mL) | [90] |
| Acalypha alnifolia Klein ex Willd. | Euphorbiaceae | Aerial parts | Aqueous fraction | 64.8 | Galantamine (0.77 μg/mL) | [80] |
| Polygonum multiflorum Thunb | Polygonaceae | Root | Ethanolic extract | 65 | Not mentioned | [69] |
| Nelumbo nucifera Gaertn. | Nelumbonaceae | Embryo | Chloroform fraction | 67.3 | Eserine (0.02 μg/mL) | [92] |
| Buchanania axillaris (Desr.) Ramamoorthy | Anacardiaceae | Aerial parts | n-Butanol fraction | 67.51 | Galantamine (0.77 μg/mL) | [68] |
| Peganum harmala L. | Zygophyllaceae | Seed | Methanolic fraction | 68.0 | Galantamine (9.4 μg/mL) | [65] |
| Atriplex laciniata L. | Amaranthaceae | Whole plant | Flavonoid fraction | 70.0 | Galantamine (52.0 μg/mL) | [104] |
| Salsola oppositifolia Desf. | Amaranthaceae | Aerial parts | Tetrahydroisoquinoline alkaloids | 70.0 | Physostigmine (0.2 μg/mL) | [90] |
| Scabiosa arenaria Forssk. | Caprifoliaceae | Flower | Buthanolic fraction | 74.0 | Eserine (0.003 μg/mL) | [79] |
| Rumex hastatus D. Don | Polygonaceae | Whole plant | Chloroform fraction | 75.0 | Galantamine (20.0 μg/mL) | [85] |
| Sophora mollis (Royle) Graham ex Baker | Fabaceae | Leaf | Methanol extract | 75.9 | Galantamine (< 1.0 μg/mL) | [105] |
| Ruprechtia apetala Wedd. | Polygonaceae | Aerial parts | Ethanol or hexane | 77.9 | Physostigmine (2.8 μg/mL) | [106] |
| Scabiosa arenaria Forssk. | Caprifoliaceae | Flower | Methanolic extract | 80.0 | Eserine (0.003 μg/mL) | [79] |
| Senna alata (L.) Roxb. | Leguminosae | Leaf | Ethyl acetate extract | 80 | Galantamine (0.37 μg/mL) | [101] |
| Carpolobia lutea G. Don | Polygalaceae | Leaf | Ethanolic fraction | 81.0 | Physostigmine (0.2 μg/mL) | [59] |
| Ochna obtusata DC. | Ochnaceae | Aerial parts | Methanolic extract | 82.2 | Galantamine (0.77 μg/mL) | [80] |
| Berberis libanotica Ehrenb. ex C.K. Schneid. | Berberidaceae | Root | Alkaloidal extract | 82.4 | Physostigmine (0.00017) | [74] |
| Searsia mysorensis (G. Don) Moffett. | Anacardiaceae | Aerial parts | n-Butanol fraction | 83.55 | Galantamine (0.77 μg/mL) | [68] |
| Illicium verum Hook.f. | Schisandraceae | Fruit | Ethyl acetate fraction | 83.75 | Galantamine (10.14 μg/mL) | [97] |
| Aloe ferox Mill. | Xanthorrhoeaceae | Leaf | 50% methanol extract | 84.0 | Galantamine (0.33 μg/mL) | [66] |
| Spatholobus suberectus Dunn. | Leguminosae | Stem | Aqueous extract | 85 | Not mentioned | [69] |
| Marrubium alysson L. | Lamiaceae | Whole plant | Methanol extract | 89.31 | Donepenzil (3.38 μg/mL) | [107] |
| Atriplex laciniata L. | Amaranthaceae | Whole plant | Saponin fraction | 90.0 | Galantamine (52.0 μg/mL) | [104] |
| Aquilegia pubiflora Wall. Ex Royle | Ranunculaceae | Leaf | Ethanolic extract | 91 | Galantamine | [108] |
| Wedelia chinensis | Asteraceae | Whole | Crude methanol extract | 93.64 | Donepenzil (9.21 μg/mL) | [98] |
| Searsia mysorensis (G. Don) Moffett. | Anacardiaceae | Aerial parts | Aqueous fraction | 93.67 | Galantamine (0.77 μg/mL) | [68] |
| Pinus nigra J.F.Arnold | Pinaceae | Needle | Essential oil | 94.4 | Galantamine (0.3 μg/mL) | [102] |
| Berberis libanotica Ehrenb. ex C.K. Schneid. | Berberidaceae | Root | Hexanic extract | 95.5 | Physostigmine (0.00017) | [74] |
| Jatropha gossypifolia L. | Euphorbiaceae | Leaf | Ethyl acetate fraction | 95.7 | Eserine (0.04 μg/mL) | [109] |
| Aquilegia pubiflora Wall. Ex Royle | Ranunculaceae | Leaf | Methanolic extract | 98 | Galantamine | [108] |
| Nonea micrantha Bioss. & Reut | Boraginaceae | Whole | Ethyl acetate | 100 | Galantamine (0.1 μg/mL) | [99] |
| Persicaria hydropiper (L.) Delarbre. | Polygonaceae | Whole plant | Aqueous fraction | 100.0 | Galantamine (0.1 μg/mL) | [94] |
| Pavetta indica L. | Rubiaceae | Aerial parts | Aqueous fraction | 100.4 | Galantamine (0.77 μg/mL) | [80] |
| Pinus nigra subsp. laricio Palib. ex Maire | Pinaceae | Needle | Essential oil | 101.5 | Galantamine (0.3 μg/mL) | [102] |
| Marrubium alysson L. | Lamiaceae | Whole plant | Non-polar fraction | 102.2 | Donepenzil (3.38 μg/mL) | [107] |
| Stemona sessilifolia (Miq.) Miq. | Stemonaceae | Root | Alkaloidal extracts | 102.6 | Huperzin A (74.5 μg/mL) | [110] |
| Illicium verum Hook.f. | Schisandraceae | Fruit | Chloroform fraction | 103.03 | Galantamine (10.14 μg/mL) | [97] |
| Centella asiatica (L.) Urb. | Apiaceae | Whole | Hydroalcohol extract | 106.55 | Physostigmine (0.076 μg/mL) | [111] |
| Persicaria hydropiper (L.) Delarbre. | Polygonaceae | Whole plant | Saponin fraction | 108.0 | Galantamine (0.1 μg/mL) | [94] |
| Trichocline reptans B.L.Rob. | Asteraceae | Aerial parts | Hexane Fraction | 111.80 | Physostigmine (2.8 μg/mL) | [106] |
| Huperzia squarrosa (G. Forst.) Trevis. | Lycopodiaceae | Aerial parts | Ethanolic extract | 112.2 | Berberine chloride (0.28 μg/mL) | [86] |
| Hemidesmus indicus (L.) R. Br. ex Schult. | Apocynaceae | Aerial parts | n-Butanol fraction | 113.5 | Galantamine (0.77 μg/mL) | [68] |
| Rumex hastatus D. Don | Polygonaceae | Whole plant | Ethyl acetate fraction | 115.0 | Galantamine (20.0 μg/mL) | [85] |
| Chenopodium quinoa Willd. | Amaranthaceae | Seed | Ethyl acetate extract | 118.91 | Galantamine (2.76 μg/mL) | [112] |
| Nelumbo nucifera Gaertn. | Nelumbonaceae | Embryo | Aqueous fraction | 119.6 | Eserine (0.02 μg/mL) | [92] |
| Ipomoea asarifolia (Desr.) Roem. & Schult. | Convolvulaceae | Leaf | Methanol extract | 120 | Galantamine (0.37 μg/mL) | [101] |
| Persicaria hydropiper (L.) Delarbre. | Polygonaceae | Leaf | Essential oils | 120.0 | Galantamine (15 μg/mL) | [113] |
| Wedelia chinensis | Asteraceae | Whole | chloroform fraction | 121.97 | Donepenzil (9.21 μg/mL) | [98] |
| Hemidesmus indicus (L.) R. Br. ex Schult. | Apocynaceae | Aerial parts | Aqueous extract | 129.43 | Galantamine (0.77 μg/mL) | [68] |
| Nardostachys jatamansi DC. | Caprifoliaceae | Rhizome | Hydroalcohol extract | 130.10 | Physostigmine (0.076 μg/mL) | [111] |
| Myristica fragrans Houtt. | Myristicaceae | Seed | Hydroalcohol extract | 133.28 | Physostigmine (0.076 μg/mL) | [111] |
| Rumex hastatus D. Don | Polygonaceae | Whole plant | Saponin fraction | 135.0 | Galantamine (20.0 μg/mL) | [85] |
| Buchanania axillaris (Desr.) Ramamoorthy | Anacardiaceae | Aerial parts | Aqueous fraction | 136.21 | Galantamine (0.77 μg/mL) | [68] |
| Carpolobia lutea G. Don | Polygalaceae | Stem | Hexane fraction | 140 | Physostigmine (0.2 μg/mL) | [59] |
| Citrus aurantifolia | Rutaceae | Leaf | Essential oil | 139 | Galantamine | [114] |
| Evalvulus alsinoides L. | Convolvulaceae | Whole plant | Hydroalcohol extract | 141.76 | Physostigmine (0.076 μg/mL) | [111] |
| Carpolobia lutea G. Don | Polygalaceae | Stem | Methanolic fraction | 142.0 | Physostigmine (0.2 μg/mL) | [59] |
| Nonea micrantha Bioss. & Reut | Boraginaceae | Whole | Chloroform fraction | 144 | Galantamine (0.1 μg/mL) | [99] |
| Blumea lacera (Burm.f.) DC. | Compositae | Leaf | Acetone extract | 150 | Galantamine (0.92 μg/mL) | [115] |
An investigation of the bioactivity of various extracts obtained from the fruits of Horsfieldia tomentosa was conducted by Idris et al. [72]. Among the four extracts of H. tomentosa, the ethyl acetate extract exhibited the highest AChE inhibition (IC50 6.0 μg/mL) and significant antioxidant activity. It showed dual but selective inhibition of cholinesterases (BuChE IC50 32.1 μg/mL). Methanol extract, n-hexane non-soluble and n-hexane soluble fractions also exhibited significant AChEI activity (IC50 9.1 μg/mL, 13.5 μg/mL, and 21.2 μg/mL, respectively) compared to the standard eserine (IC50 27.53 μg/mL). This multipotency makes this plant a potential candidate for AD therapeutic drug discovery.
Traditionally, Sarcocephalus latifolius is a plant used in folklore medicine to treat malaria, jaundice, dysentery, hypertension, inflammation, aches and snake bites. It is also reported to have antioxidant and AChEI activities. In an experiment by Atolani et al. [89], fatty acid methyl esters from the leaves and fruits of S. latifolius were isolated by a one-step extraction and transesterification process. Although the leaves had higher extraction yield, they showed very weak AChEI activity, compared with fruit (IC50 29.14 mg/mL) and the reference, donepezil (127.70 mg/mL). Saad et al. [57] reported the AChEI potential of leaf extracts of Citharexylum spinosum. Though all extracts showed pronounced activity, the hexane-benzene fraction showed the highest inhibition (IC50 0.08 μg/mL), comparable to the reference compound donepezil (IC50 0.05 μg/mL). Other extracts such as n-butanol, hydro-alcohol (50%), chloroform, hexane and ethyl acetate fractions also showed strong inhibitory potential, with IC50 values ranging from 0.6–4.8 μg/mL, making C. spinosum an important plant in AD therapy.
Hexane and ethanolic extracts of seed and pulp/peel of Byrsonima sericea, utilized in traditional medicine of the Amazon region for the treatment of fever, skin infections, asthma, respiratory diseases and gastrointestinal problems, were evaluated for AChEI action [73]. The researchers found that hexane extract of the seed as well as ethanolic extracts of seed and pulp showed marked inhibition against AChE (IC50 6.62 μg/mL, 6.04 μg/mL, and 9.04 μg/mL, respectively), while hexane extract of pulp showed moderate (IC50 10.92 μg/mL) AChE inhibition, with reference to the standard galantamine (IC50 1.02 μg/mL).
Hypericum spp. has traditionally been used to treat mental disorders, depression, diabetes, jaundice, arthritis, and bladder complications [63]. It has been presented that hydro alcohol extract of H. perforatum reduced the ACh breakdown rate by strongly inhibiting AChE activity, with an IC50 value of 4.32 μg/mL [63]. Furthermore, acetone, chloroform, methanol and ethanol extracts of H. heterophyllum leaves significantly decreased AChE activity (IC50 11.07 μg/mL, 12.33 μg/mL, 13.99 μg/mL and 16.32 μg/mL, respectively), suggesting the presence of potential in AChEI phytochemical in Hypericum spp. [77].
Hemidesmus indicus (Apocyanaceae) is an important medicinal plant with wound-healing properties and is used against ulcers, fever, headache, eye disease, asthma, rheumatism, leucorrhoea, diarrhoea, prostatitis, neuralgia, nephritic disorders, urticaria, psoriasis, itching, and dyspepsia [68]. As part of an experiment, Penumala et al. [68] aerial parts of H. indicus were extracted with trichloromethane, 90% methanol, n-butanol and water and tested for AChEI activity. Results showed that trichloromethane and methanol extracts inhibited AChE moderately with IC50 values of 28.14 μg/mL and 48.64 μg/mL, respectively, compared to the standard galantamine (IC50 0.77 μg/mL). The other two extracts showed weak inhibition (IC50 > 100.0 μg/mL).
In a study by Ali et al. [65], whole plant extracts of Angelica decursiva were tested for anti-AChE activity in different fractions. Aqueous, butanolic, ethyl acetate, chloroform and methanolic fractions showed substantial inhibitory effects with IC50 values of 2.6 μg/mL, 6.0 μg/mL, 9.7 μg/mL, 13.7 μg/mL, 16.6 μg/mL, respectively, suggesting that it might contain bioactive compounds that can treat AD. Esenbeckia leiocarpa Engl. is an ornamental tree with a variety of secondary metabolites, including quinolinic, quinolonic and indolic alkaloids and furocoumarins, making it a therapeutically important plant. Using the Ellman colorimetric technique, Cardoso-Lopes et al. [62] tested the AChEI activity of ethanol extract, hexane and alkaloid fractions of E. leiocarpa. The alkaloidal and hexane fractions showed strong inhibition (IC50 1.6 μg/mL and 6.0 μg/mL, respectively), while ethanol extract exhibited moderate inhibition to AChE (50.7 μg/mL).
Huperzia spp. (Lycopodiaceae) have been used to treat a wide range of cognitive and neurological disorders in China for over 1,000 years [1]. A steep surge in research focusing on cholinesterase inhibitory properties of Huperzia spp. has been reported in the past decade following the isolation of the alkaloid Huperzine A from H. serrata. In a study, Armijos et al. [61] reported the strong AChEI potential of the alkaloid fraction of H. tetragona aerial parts with an IC50 value of 0.9 μg/mL. H. brevifolia and H. compacta displayed moderate activity (IC50 39.6 μg/mL and 62.4 μg/mL, respectively) compared to the standard donepezil (IC50 0.036 μg/mL). In the study by Ohba et al. [70], the alkaloid-enriched fraction of H. serrata aerial parts, whose primary component was Huperzin A, displayed an IC50 value of 5.96 μg/mL. In a similar study, Tung et al. [86] analyzed the AChEI potential of ethyl acetate fraction, buthanolic fraction and ethanolic extract of H. squarrosa. Results suggested that while ethyl acetate and butanolic fractions showed moderate AChE inhibition (IC50 23.4 μg/mL and 50.1 μg/mL, respectively), ethanolic extract showed week inhibitory potential (IC50 112.2 μg/mL). Even with the clinical evidence of the AChEI potential of Huperzin A, researchers are still focused on the pharmacological properties of Hupperzia spp. [1, 116].
Table 1 summarises screening studies on medicinal plants, including their scientific names, family, plant part, type of extract or fraction, IC50 value of AChE inhibition and positive control. This table gives an overview of the species under consideration, which are arranged in the ascending order of IC50 values of various extracts. AChEI activity of plant extracts was divided into four categories based on their IC50 values (Figure 2): strong activity (IC50 < 10 μg/mL), moderate activity (10–50 μg/mL), low activity (50–100 μg/mL) and very weak activity (> 100 μg/mL). As per the abovementioned studies, 45 plant extracts (from 28 species) were found to have strong inhibition against AChE (Figure 2), with IC50 values ranging from 0.08 μg/mL for essential oil extract from the fruit peel of Myrciaria floribunda [100] and hexane-benzene fraction of Citharexylum spinosum L. leaf extract [57] to 10.0 μg/mL for the root methanolic extract of Zanthoxylum davyi Waterm. (Table 1) [60]. Moderate activity was displayed by 85 plant extracts (from 60 species; Figure 2) with IC50 values varying from 10.8 μg/mL for the total Alkaloids fraction of stem extract of Uncaria rhynchophylla (Miq.) Miq. ex Havil. [76] to 50.0 μg/mL for the aqueous extract of Salvia miltiorrhiza Bunge roots (Table 1) [69]. Forty-eight plant extracts (from 34 species) have been identified in this review with low activity, whose IC50 values vary from 50.1 μg/mL for butanolic fraction of Huperzia squarrosa (G. Forst.) Trevis. f. [86] to 100.0 μg/mL for the aqueous fraction of Persicaria hydropiper (L.) Delarbre. (Table 1) [94]. This review also assessed 27 plant extracts (from 23 species) having very weak inhibition against AChE (Figure 2), with IC50 values ranging from 100.4 μg/mL to 150.0 μg/mL (Table 1).
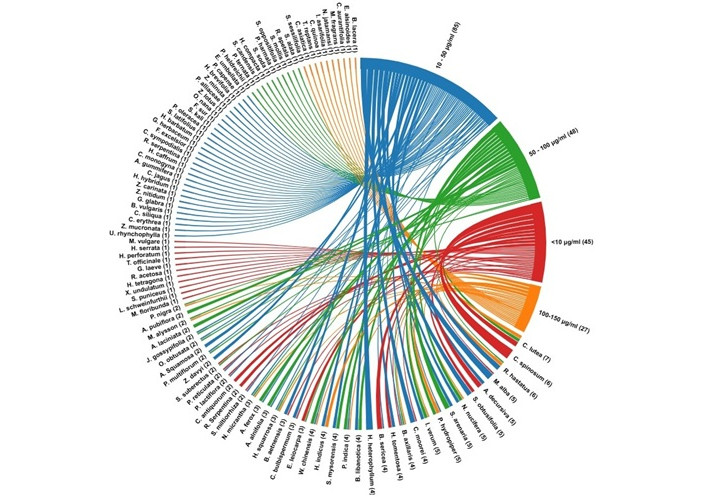
Chord diagram showing the AChE inhibition (4 larger arcs on the right side of the chord diagram) of various plant extracts (smaller arcs on the left side of the chord diagram). The AChE inhibition of extracts is given in terms of IC50 values such as (1) strong inhibition: IC50 < 10 µg/L; (2) moderate inhibition: IC50 10–50 µg/L; (3) low inhibition: IC50 50–100 µg/L; (4) very weak inhibition: IC50 100–150 µg/L. The chord diagram was created using RAW Graphs (rawgraphs.io).
Further analysis of the above studies suggested the importance of plant parts and solvents used in extraction. Different solvent extracts of the same plant part showed varying inhibitory concentrations, suggesting the isolation of bioactive molecules responsible for AChE inhibition in specific solvents, depending on their polarity and other factors. For instance, Suciati et al. [71] reported that ethanol extract, n-butanol and ethyl acetate fraction from roots of Rauvolfia serpentina (L.) Bth. ex Kurz presented different inhibitory concentrations. While n-butanol and ethyl acetate fractions displayed strong inhibition (IC50 5.99 μg/mL and 7.46 μg/mL, respectively), ethanol extract presented moderate inhibition (IC50 23.62 μg/mL) (Table 1). Similarly, different solvent extracts or fractions from the same plant parts of species such as Acalypha alnifolia Klein ex Willd., Aloe ferox Mill., Berberis libanotica Ehrenb. ex C.K. Schneid., Crinum bulbispermum (Burm. f.) Milne-Redh. & Schweick., Polygonum multiflorum Thunb, Pycnostachys reticulata (E.Mey.) Benth., etc., showed variable inhibitory capacity (Table 1).
Also, different plant parts extracted in the same solvent or fraction of various plant parts exhibited varying inhibitory potential against AChE, suggesting the selective production and accumulation of phytochemicals in different plant parts. For example, the methanolic extract of pulp and seeds of Annona Squamosa L. presented varying anti-AChE activity with IC50 values of 18.82 μg/mL and 22.31 μg/mL, respectively (Table 1) [83]. Similarly, ethyl acetate extract of the bulb and root of Crinum bulbispermum (Burm. f.) Milne-Redh. & Schweick exhibited significant variation in their AChEI potential. While the ethyl acetate extract of the bulb displayed notable inhibitory activity with an IC50 value of 2.1 μg/mL, that of the root showed moderate activity (IC50 39.3 μg/mL) [65].
Several plant species that produce a variety of bioactive compounds have been studied for anti-AChE activity and might be used to develop novel anti-AD medicines. Extracts from 100 species expanding in 47 plant families and 80 genera are categorized and discussed in this review (Table 1, Figure 3). Amaryllidaceae, Fabaceae, Amaranthaceae, Lycopodiaceae, Anacardiaceae, and Polygonaceae were the most common families with seven, six, five, five, five, and five species, respectively (Figure 3). Crinum bulbispermum (Burm. f.) Milne-Redh. & Schweick., Crinum jagus (J. Thomps.) Dandy, Crinum moorei Hook.f., Hippeastrum barbatum Herb, Hippeastrum hybridum, Zephyranthes carinata Herb., and Zephyranthes minuta (Kunth) D.Dietr. are the seven species of Amaryllidaceae that has presented significant AChEI activity. Most of the plant extracts from Amaryllidaceae exhibit strong to moderate inhibition, with IC50 values ranging from 2.1 μg/mL to 39.2 μg/mL (Table 1). Notably, extracts of all these plants except for H. hybridum came from their bulbs, indicating the presence of AChEI phytochemicals in the bulbs of Amaryllidaceae species. Amaryllidaceae is a monocot family comprising around 1,100 perennial bulbous, perennial plants rich in isoquinoline alkaloids, known as the Amaryllidaceae alkaloids [117]. Studies have shown that Amaryllidaceae alkaloids are responsible for the anti-AChE properties of this family [118]. Similarly, several lead molecules with AChEI potential have been identified and isolated from various plants, which are discussed further in section 4.
Plant-based medicines have gained more popularity in recent years as an alternative to synthetic drugs in treating various illnesses. Similar is the case of AD, where phytomedicines are being intensely studied as an alternative approach for the holistic treatment of AD [119]. Herbal medicines have been used in various traditional medical systems to enhance memory and learning. Studies have shown that several phytochemicals, including flavonoids, vitamin E, vitamin C, and beta-carotene, have antioxidant properties and are known to enhance brain function by reducing oxidative stress [120]. As there is an urgent need for more efficient drugs with least or no side effects to treat AD, phytochemicals with neuroprotective activity and minimal side effects are being studied extensively to find a compound with the potential to become a multi-targeted drug that interacts with multiple targets of AD [121, 122]. This part of the review focuses on phytocompounds with AChEI activity for treating AD. Numerous phytochemicals coming under the category of alkaloids, polyphenols, flavonoids and terpenoids are reported to have AChE inhibition potential in addition to antioxidant, anti-inflammatory and neuroprotective properties [123, 124], recent studies on which are discussed here.
Alkaloids are a group of low molecular weight nitrogen-containing phytochemicals derived biosynthetically from amino acids, leading to diverse chemical structures [125]. They exhibit a wide range of biological activities and are used in therapies such as pain reduction (opiate alkaloids) and chemotherapy (vinblastine, vincristine and taxane). Alkaloids are the main category of phytochemicals with AChEI potential. Galantamine extracted from the Galanthus species is the first and only FDA-approved alkaloid for treating AD. Different classes of alkaloids, including indole alkaloids, isoquinoline alkaloids, steroid alkaloids, terpenoid alkaloids and β-carboline alkaloids, have been studied for their neuroprotective role and potential in AD treatment [126].
Indole alkaloids constitute a major class of alkaloids that are derived from tryptophan and secologanin, which include compounds such as reserpine, ajmalicine, vinblastine, physostigmine and yohimbine and have therapeutic properties [127]. Physostigmine is an indole alcohol with AChEI potential isolated from Physostigma venenosum. Phenserine and tolserine are its derivatives, which also possess AChEI activity. Physostigmine is no longer used in AD treatment due to its side effects and short half-life [128, 129]. Ajmalicine and reserpine are indole alkaloids with AChEI potential, isolated from Rauwolfia serpentina Benth. Ex Karz [130]. They are also used to control blood pressure due to their antihypertensive potential [131]. Reserpine turned out to be a dual cholinesterase inhibitor of AChE and BuChE with IC50 values comparable to galantamine. Both ajmalicine and reserpine also inhibited Aβ42 fibril formation and other AD targets such as BACE-1 and MAO-B [132, 133]. Uleine is another indole alkaloid isolated from Aspidosperm ulei, and possesses high AChEI potential (IC50 0.4 µM).
Similarly, harmine and harmaline are β-carboline-type indole alkaloids isolated from Peganum harmala L. [134]. Being natural inhibitors of MAO, harmine and harmaline are used in ayahuasca, a drink used by South American people that has anxiolytic and antidepressant effects [135]. Studies have shown that these alkaloids possess AChEI potential and antioxidant, anti-inflammatory and MAO-A inhibitory properties. They also have the potential to cross the BBB [55]. They enhance cholinergic function by multiple pathways, including inhibition of AChE, limiting maleic dialdehyde production, antioxidant defence by increasing the activity of glutathione peroxidase and superoxide dismutase, reducing inflammation by suppressing TNFα, nitrous oxide and myeloperoxidases [136].
Isoquinoline alkaloids are one of the largest groups of plant alkaloids; many are potential therapeutic agents for various diseases. Protoberberine and aporphine types are the most common types of isoquinoline alkaloids [137]. Galantamine, lycorine, ungeremine, berberine, epiberberine and palmatine are some of the isoquinoline alkaloids with AChEI properties. Berberine and palmatine are protoberberine-type quaternary isoquinoline alkaloids derived from tyrosine. Both compounds are seen in Chinese memory booster herbs such as Corydalis rhizoma and Coptidis rhizome [138]. Berberine is extensively studied as a potential drug for various ailments, as it has a plethora of bioactivities such as antibacterial, antiviral, anti-inflammatory, anticancer and antihyperglycemic activity [139, 140]. Studies have shown that berberine has notable inhibitory activity against AChE (IC50: 0.5–0.7 µM) and BuChE (IC50: 30.7 µM) and also has the potential to inhibit MAO-A enzyme [138, 141, 142]. As a safe, non-toxic chemical capable of reducing AD risk factors such as diabetes and atherosclerosis, berberine can be orally administered as an anti-AD drug [143]. Pseudoberberine and pseudocoptisine are also isoquinoline alkaloids with AChEI properties [144, 145].
Palmatine is reported to be an efficient inhibitor of AChE (IC50 0.46–1.69 µM) compared to BuChE (IC50 > 100 µM) [138, 141, 146]. As per recent reports, palmatine has inhibitory potential against MAO-A [147] and can also cross the BBB, causing changes in the hippocampus and cerebellum [148]. A synergistic effect of parallel administration of berberine and palmatine was reported by [138], suggesting that the positively charged nitrogen in these alkaloids can bind to the gorge of the active site and thereby inhibit the enzyme [149].
Chelerythrine is a benzophenanthridine isoquinoline alkaloid isolated from Chelidonium majus L. that has dual cholinesterase activity against AChE (IC50 1.54 µM) and BuChE (IC50 10.34 µM) [150]. Chelerythrine is also a potential inhibitor of Aβ1–40 aggregation and deaggregation of preexisting Aβ1–40 aggregates [146]. It has also shown selective inhibition against the human MAO-A enzyme [151]. Extracts of Zanthoxylum rigidum Humb. Et Bonpl. Ex Willd yielded two alkaloids, avicine and ntidine, with dual cholinesterase inhibitory activity against AChE and BuChE. Being mixed-type inhibitors, they can bind at the catalytic and PAS of the enzymes and inhibit ChE-induced Aβ1–40 aggregates. Like chelerythrine, avicine and nitidine also showed inhibitory activity against MAO-A [152]. Groenlandicine and jatrorrhizine are also isoquinoline alkaloids isolated from Coptis chinensis Franch., which possess BACE-1 Inhibitory activity in addition to AChEI potential [153].
Amaryllidaceae alkaloids are a special class of alkaloids restricted to the family Amaryllidaceae. Galantamine is one of the most important Amaryllidaceae alkaloids isolated from Galanthus woronowii. It is an FDA-approved drug for treating AD and is a selective inhibitor of AChE compared to BuChE. The Narcissus genus gained significant attention in phytochemical and pharmaceutical research due to its wide range of alkaloidal content. Galantamine is now isolated on an industrial scale from the bulbs of Narcissus spp. [117, 154]. Ungeremine and lycorine are alkaloids with a moderate AChEI potential isolated from Narine bowdenii W. Watson and Narcissus pseudonarcissus [118]. Around 600 Amaryllidaceae alkaloids have been isolated so far, of which the Narcissus genus alone contributes more than 100 [155, 156]. Haemanthamine is yet another type of a β-crinane-type Amaryllidaceae alkaloid in many Narcissus species. Several reports reveal the AChEI activity and in vitro cytotoxic of haemanthamine against cancer cell lines such as HeLa, MCF7, HepG2, and A549 [11, 157].
Lycopodium alkaloids are another category of alkaloids found in the family Lycopodiaceae, and they possess an unusual tetracyclic ring structure. Carinatumins A and B isolate from Lycopodium carinatum Desv. ex Poir. possess notable AChEI activity with IC50 values of 4.6 µM and 7.0 µM, respectively [128, 158]. Lycojaponidine A is another lycopodium alkaloid with a high AChEI capacity similar to galantamine and hence, a potential drug for AD treatment [128]. Huperzine A is one of the highly potent and well-studied AChE inhibitor alkaloids isolated from Huperzia serrata. It is a reversible inhibitor of AChE with activity comparable to that of galantamine and physostigmine [159–161]. It has dual cholinesterase inhibitory activity but is more selective to AChE in different brain parts, including the cerebellum, cortex, hippocampus, and hypothalamus. It has increased bioavailability and can cross the BBB [159–161]. Being a mixed-type inhibitor, huperzine A also regulates amyloid beta precursor protein metabolism and inhibits Aβ aggregation, thereby reducing Aβ-associated neurotoxicity [162, 163].
Another category of alkaloids is the triterpenoid steroidal alkaloids. Several of them, including lyrcanone, hyrcamine, and buxidine, have been shown to have notable AChEI potential [127]. There are still many alkaloids yet to be studied and many to be clinically tested, which could be potential drugs for the treatment of AD and other neurodegenerative diseases.
Caffeine is a plant alkaloid found in coffee (Coffea arabica), which is a central nervous system stimulant [164]. Studies have shown that caffeine is a non-competitive inhibitor of AChE. Though its activity is moderate compared to donepezil and galantamine, it can be administered in higher doses as it is less toxic [165]. Recent research shows that habitual caffeine intake can increase cognitive response in females with AD [166]. Also, short-term intake of a combination of caffeine and caffeic acid in moderate concentration (50 mg/kg body weight) can reduce AD symptoms by lowering lipid peroxidation, increasing antioxidant status and inhibiting AChE, arginase and adenosine deaminase activity, thereby improving brain activity [167].
Polyphenols are phenylpropanoid secondary metabolites produced by plants as a defence mechanism against pathogens or stress. Polyphenols are commonly classified as flavonoids and nonflavonoids. Flavonoids include flavonols, flavones, isoflavones, flavanones, anthocyanidins and chalcones, while nonflavonoids include phenolic acids (hydroxybenzoic acids, cinnamic acids) and phenolic amides [168]. Numerous studies have been published emphasizing the potential of polyphenols in suppressing the onset and advancement of several diseases [169, 170]. The interaction of phenolic compounds with active site amino acid residues of AChE is characterized by hydrogen bonding, hydrophobic interactions, and π-π stacking [171]. Moreover, the presence of multiple hydroxyl groups enhances the binding capacity of phenolic compounds and consequently, their AChEI potential [172].
Flavonoids are a large class of polyphenolic secondary metabolites widely found in fruits, vegetables, herbs, and some beverages [173]. These are of interest due to their health-promoting effects, such as antioxidant, anti-inflammatory, anticancerous and antimutagenic properties, which increase their demand in pharmaceutical, neutraceutical, cosmetic and medicinal fields [174]. Many flavonoids are potential inhibitors of enzymes, such as lipoxygenase, cyclo-oxygenase, xanthine-oxidase and cholinesterases [173]. There are six major classes of flavonoids based on the position of benzoid and its modification, which include flavonols (quercetin, myricetin, kaempferol), flavones (apigenin, luteolin, chrysin), isoflavones (daidzein, genistein), flavanones (naringenin, hesperetin), anthocyanidins (cyanidin, delphinidin) and chalcones (flavokawin, cardamonin) [173].
Flavonoids are secondary metabolites that can reverse AD symptoms and improve memory and learning via multiple pathways, including AChE—choline acetyltransferase (ChAT) balancing and modulation of G protein-coupled receptor 30 (GPR30) [175, 176]. Genistein and quercetin are two flavonoids that inhibit AChE activity and enhance ChAT activity, leading to increased levels of ACh in the hippocampus and thereby improving cholinergic neurotransmission metabolites [176]. Flavonoids such as quercetin, kaempferol, luteolin and chrysin, which possess high antioxidant activity, reduce oxidative stress-induced AD by scavenging reactive oxygen species (ROS) as well as activating antioxidant enzymes such as catalase and superoxide dismutase [177–179].
In a study to find the AChEI potential of selected flavonoids, quercetin and tiliroside demonstrated remarkable AChEI potential of IC50 19.8 μM and 23.5 μM, respectively [180]. However, another study focused on leishmanicidal and AChEI activities of flavonoids isolated from the beans of Dimorphandra gardneriana revealed that rutin and quercetin displayed remarkable leishmanicidal activity and AChEI activity compared to the standard physostigmine [181]. Dzoyem and Eloff [182] reported that flavonoids isolated from Dorstenia and Polygonum species such as 2’,4’-dihydroxy-3’,6’-dimethoxychalcone, 3 6-8-diprenyleriodictyol, isobavachalcone, 4-hydroxylonchocarpin, 6-prenylapigenin showed significant AChEI activity with values ranging from 5.93–8.76 μg/mL which is comparable to the positive control eserine (IC50 4.94 μg/mL). In addition, 3 6-8-diprenyleriodictyol, isobavachalcone and 6-prenylapigenin also showed lipoxygenase inhibitory activity.
On screening a series of flavonoids for their AChEI activity, Balkis et al. [183] found that baicalein from Scutellaria baicalensis roots had the highest AChEI potential with an IC50 value of 0.61 μM as compared to positive control tacrine (IC50 25.4 μM). An investigation of the enzyme kinetics revealed that baicalein is a mixed-type inhibitor. Significant inhibitory activity was also shown by apigenin, cyanidin, chrysin, kaempferol, myricetin, quercitrin, perlargonidin, (–)-epigallocatechin gallate (EGCG), and galangin with an IC50 ranging from 3.05 μM to 19.1 μM. Other flavonoids such as baohouside 1, dephinidin and melvidin displayed relatively low inhibition with IC50 ranging from 41.1–89 μM. Sevindik et al. [184] isolated four flavonoids with remarkable cholinesterase inhibitory potential from the aerial part of Achillea millefolium. Of the four, 6-OH-luteolin 7-O-β-D-glucoside displayed the highest AChE (IC50 1.65 μM) and BChE (IC50 1.97 μM) inhibitory activity, suggesting its potential to become a novel therapeutic drug for AD treatment.
EGCG, a flavonoid in green tea, has significant AChEI activity [185]. It has also shown inhibition of tau-phosphorylation, antioxidant, anti-inflammatory and antiapoptotic properties, altogether contributing to its neuroprotective properties [185]. Naringin is a flavanone that inhibits AChE activity, neuroinflammation in stress-prone brain regions, nitric oxide-induced stress and oxidative stress [186].
Curcumin is the prominent polyphenolic component in Curcuma longa that possesses antimicrobial, antiviral, antioxidant, anti-inflammatory and anticarcinogenic properties [187, 188]. Curcumin is used in the treatment of various diseases such as AD, psoriasis, multiple myeloma, myelodysplastic syndrome, pancreatic cancer and AIDS [188–190]. A recent in vitro study reported the AChEI potential of curcuminoids. Among curcuminoids, the highest AChEI potential was observed in bisdemethoxy curcumin (IC50 2.14 μM), followed by demethoxycurcumin (IC50 19.7 μM). Curcumin showed comparatively lower activity (IC50 51.8 μM). While bisdemethoxycurcumin showed a low inhibitory activity against BuChE (IC50 67.2 μM), curcumin and demethoxycurcumin had no inhibitory potential against the same [191]. Two studies recently reported the AChEI potential of curcumin in rat models. Wolkmer et al. [192] reported that Wistar rats fed 0.1 mL/kg body weight of curcumin daily showed inhibition of AChE and improved immunological response. In another study, Akinyemi et al. [193] reported AChE inhibition mediated memory improvement in cadmium-exposed albino rats fed with curcumin (12.5 mg/kg) for 7 days.
The dual cholinesterase inhibitory potential of resveratrol oligomers isolated from Vitis amurensis was reported by Jang et al. [194]. The resveratrol oligomers (10 μg/mL), vitisin A and heyneanol A showed inhibition against AChE, BuChE and Aβ aggregation in a dose-dependent manner. In another study, male Wistar rats treated with 10 mg/kg of resveratrol showed a remarkable AChE activity reduction [195]. Further studies showed a reduction in AChE activity in synaptomes of the cerebral cortex in 10 mg/kg resveratrol-treated diabetic rats [196]. Another therapeutic approach with pyridoxine-resveratrol hybrids showed a mixed inhibition pattern (IC50 1.56–2.11 μM) by simultaneously binding to the catalytic and PAS of AChE [197]. Both curcumin and resveratrol are promising AChE inhibitors currently under phase 3 clinical trials against AD [120].
Cinnamic acid is a polyphenolic compound found abundantly in fruits, spices and vegetables such as cinnamon (Cinnamomum cassia), grapes, citrus fruits, cocoa, celery, cruciferous vegetables and spinach [198, 199]. Numerous studies have reported its antioxidant, anticancer, anti-inflammatory and antidiabetic properties [198, 200, 201]. They can be administered orally as they are readily absorbed by the small intestine [202]. Elufioye et al. [203] reported the AChE (6.51 μg/mL) and BuChE (9.07 μg/mL) inhibitory potential of 2,3-dimethyl derivative of cinnamic acid (omifoate A), isolated from the leaves of pycnanthus angolensis (Welw.) Warb. Lan et al. [204] reported the remarkable AChEI potential (IC50 8.6 nM) of a novel cinnamic acid derivative, E)-4-(3-(3,4-dimethoxyphenyl) acrylamido)-1-(3-methylbenzyl) pyridin-1-ium bromide with over threefold higher inhibitory potential than donepezil. It also showed inhibition of Aβ aggregation, neuroprotection against amyloid-induced toxicity, and the ability to penetrate the BBB, making it a good candidate for AD therapy.
Terpenes are simple hydrocarbons composed of multiple isoprene units, representing one of the largest and most diverse classes of secondary metabolites synthesized by plants [205]. Terpenoids are terpene derivatives with different functional groups. Based on the number of isoprene units that form the parent terpene, terpenoids can be classified into hemiterpenoids (one isoprene unit), monoterpenoids (two isoprene units), sesquiterpenoids (three isoprene units), diterpenoids (four isoprene units), sesterterpenoids (five isoprene units), triterpenoids (six isoprene units) and tetraterpenoids (eight isoprene units) [206, 207]. Therapeutic applications of terpenoids owe to their wide range of biological activities, such as antioxidant, anti-inflammatory, antimicrobial, anticancer, anti-hyperglycaemic, anti-cholinesterase and immunomodulatory activities [208]. For example, Taxol and artemisinin are terpene-based drugs used as anticancer and anti-malarial medications, respectively [209–211]. The anti-cholinesterase activity of terpenoids has recently gained much attention. Numerous studies have reported the AChE and BuChE inhibitory and neuroprotective potential of terpenoids.
In a study conducted by Dohi et al. [212], for screening terpenoids from commercial essential oils, 1,8-cineole (IC50 15 μg/mL) and α-pinene (IC50 22 μg/mL) showed higher AChEI activity compared to others such as estragole and limonene (IC50 > 100 μg/mL). Wojtunik-Kulesza et al. [213] reported the remarkable AChEI potential of farnesene compared to other monoterpenes tested in a Marston assay.
In an attempt to evaluate the AChEI potential of sesquiterpenoids isolated from Cynara cornigera L., Hegazy et al. [214] found that a chlorinated sesquiterpene lactone, cornigeraline A, had the highest AChEI potential (IC50 20.5 µM). Due to the presence of two hydrophobic moieties in the nucleus, it may cross the BBB [53]. Other sesquiterpenes such as sibthropine (IC50 35.8 µM), 3-hydroxy-grosheimin (IC50 30.5 µM), grosheimin (IC50 61.8 µM), solstitalin A (IC50 25.7 µM), 13-chlorosolstitialine (IC50 62.1 µM), and cynaropicrin (IC50 31.3 µM) also displayed moderate AChE inhibition [53, 214]. Alarcón et al. [215] isolated nine dihydro-β-agarofuran sesquiterpenes from the aerial parts and seeds of Maytenus disticha (Hook.f.) Urb. and Euonymus japonicus Thunb. They were all weak but selective AChE inhibitors, with IC50 values ranging from 70–381 µg/L.
Artemisinin (IC50 103.9 µM) from Artemisia annua and quinanol A (IC50 100.8 µM) from Aquilaria sinensis (Lour.) Gilg, are sesquiterpenes with moderate AChEI potential [216, 217]. In a recent study, Zardi-Bergaoui et al. [218] isolated caryophyllene-type sesquiterpenes from aerial parts of Pulicaria vulgaris Gaertn. Among them, two compounds namely, (1S,5Z,9R,11S)-12,14-dihydroxycaryophylla-2(15),5-dien-7-one and (1S,6R,9S,11R)-13,14-dihydroxycaryophyll-2(15)-en-7-one displayed higher AChEI potential (IC50 25.8 μM and 40.0 μM respectively) compared to other compounds. Another sesquiterpenoid, Megatigma-7,9-diene-1,4-epoxy-2-hydroxy-10-carboxylic acid with high AChEI potential (IC50 9.3 μM), was isolated by Liu et al. [219] from Lycopodiastrum casuarinoides (Spring) Holub.
Diterpenes such as 12-O-demethylcryptojaponol and 6α-hydroxydemethylcryptojaponol, isolated from Caryopteris mangolica Bunge, inhibited human erythrocyte AChE, with an IC50 value of 50.8 μM and 19.2 μM, respectively, suggesting their potential role in AD therapy and the importance of C6 hydroxyl group in the inhibition of AChE [220].
Tanshinones are a category of abietane-type diterpenes isolated from Salvia species, which have high permeability across the BBB. Tanshinone from the roots of Salvia glutinosa L. and Salvia yangii B.T.Drew, namely 15,16-dihydrotanshinone was found to be a moderate inhibitor of AChE (IC50 35.8 μM). While other tanshinones isolated from the same plants, such as cryptotanshinone, Miltirone, 1β-hidroxicryptotanshinone, and 1,2-didehydromiltirone are inactive inhibitors of AChE [221]. Lycocasuarinone A is another abietane-type diterpene isolated from Lycopodiastrum casuarinoides, that inhibits (IC50 26.8 μM) AChE [219].
Numerous serratene-type triterpenes were extracted from Lycopodiastrum casuarinoides, of which 26-nor-8-oxo-21-one-α-onocerin showed remarkable inhibition of AChE (IC50 1.01 μM) compared to other compounds [219]. Nguyen et al. [222] isolated twelve serratene-type terpenes from Lycopodiella cernua. Four among the twelve, namely 3β,21α-diacetoxyserratan-14β-ol (IC50 0.91 μM), 3β,21β,29-trihydroxyserrat-14-en-3β-yl p-dihydrocoumarate (IC50 1.69 μM), 3β,14α,15α,21β-tetrahydroxyserratan-24-oicacid-3β-yl-(40-methoxy-50-hydroxybenzoate) (IC50 9.98 μM), and 21β-hydroxyserrat-14-en-3,16-dione (IC50 10.67 μM) exhibited strong inhibition against AChE [208].
Ado et al. [223] revealed the AChEI potential of euscaphic acid, arjunic acid and ursolic acid isolated from the leaves of Callicarpa maingayi K & G. They exhibited IC50 values of 35.9 μM, 37.5 μM, and 21.5 μM, respectively. Similar AChEI activity was reported by Jamila et al. [224] in triterpenoids isolated from Garcinia hombroniana Pierre 2-hydroxy-3α-O-caffeoyltaraxar-14-en-28-oic acid, betulin and betulinic acid. Among the three, Pierre 2-hydroxy-3α-O-caffeoyltaraxar-14-en-28-oic acid showed the highest AChEI potential with an IC50 value of 13.5 µM, followed by betulinic acid (IC50 24.2 µM) and betulin (IC50 28.5 µM).
Trichilia lactone D5, rohituka 3 and dregeanin DM4 are three limonoids isolated from the seeds of Trichilia welwitschia. All three showed AChE inhibition at varying levels (IC50 19.13 μM, 34.15 μM, and 45.69 μM, respectively) [53]. Colocynthenin A and colocynthenin C are cucurbitane-type triterpenes isolated from the fruits of Cirtrullus colocynthis L. They possess high AChEI potentials of 2.6 μM and 3.1 μM, respectively [225].
Another AChEI triterpenoid, 3β-Hydroxy-24-nor-urs-4(23)-12-dien-28-oic acid obtained from Patrinia scabiosaefolia showed an IC50 value of 10.0 μM. In another research, Liu et al. [226] isolated three pentacyclic triterpenes from the roots and breaches of Malpighia emarginata DC such as norfriedelin A, norfriedelin B and norfriedelin C. On analysis of their AChEI potential, norfriedelin A and B showed better activity (IC50 10.3 μM and 28.7 μM, respectively) than norfriedelin C (IC50 > 50 μM). Isolation and identification of the lead molecules from plant extracts and their structure-activity relationship studies may facilitate decision-making and selection of potential phytochemicals for drug discovery to aid in AD therapy.
AD therapy turned out to be highly challenging due to its multifactorial etiolgy and complex pathophysiology. Despite having 3 drugs that are already approved by the FDA for AD treatment, scientists are in quest of developing anti-AD drugs with minimal toxicity and side effects. This review provides a thorough overview of various medicinal plants, their extracts and phytochemicals that could be potential AChE inhibitors. Though numerous medicinal plants have AChEI properties, some families, such as Amaryllidaceae, Fabaceae, Lycopodiaceae and Amaranthaceae, have more species with anti-AChE potential. However, a drastic variation can be seen in the AChEI activity of plant extracts regardless of the family, genus, predominant phytochemical or the extracting solvent. As per the literature review and analysis, a plethora of secondary metabolites, including alkaloids, phenolics, flavonoids and terpenoids, reportedly demonstrated cholinesterase inhibitory activity. Besides, many of them show a multitarget approach in AD therapy by inhibiting AChE, BuChE, MAO-A, BACE-1, Aβ aggregation, tau phosphorylation, and an ability to cross BBB. Most of them also possess antioxidant and anti-inflammatory properties, which are also helpful in minimising oxidative stress and inflammation mediated neurodegeneration. Certain phytochemical combinations (such as berberine and palmaline) have exhibited synergistic activity against AChE. One limiting factor here would be the limited bioavailability, which can be addressed by plant tissue culture and elicited phytochemical production. As conventional therapies only give symptomatic relief of AD, alternative therapies are being largely studied, and several phytochemicals, including curcumin, are under clinical and pre-clinical trials, which may serve as potential drug candidates for the treatment of AD. These bioactive compounds can be isolated and modified to maximise efficiency, potentially revolutionising AD therapy.
Ach: acetylcholine
AChE: acetylcholinesterase
AChEIs: acetylcholinesterase inhibitors
AD: Alzheimer’s disease
Aβ: β-amyloid
BACE-1: beta site amyloid precursor protein cleaving enzyme 1
BBB: blood-brain barrier
BuChE: butyrylcholinesterase
ChAT: choline acetyltransferase
EGCG: Epigallocatechin gallate
FDA: Food and Drugs Administration
GPR30: G protein-coupled receptor 30
MAO: monoamine oxidase
PAS: peripheral anionic site
DAV: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. DTT: Conceptualization, Writing—review & editing, Supervision. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
All datasets generated or analyzed during this study are included in the manuscript and references.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Neha Kamboj ... Rahul Kumar
Suvendu Ghosh ... Debosree Ghosh
Apoorva A. Bankar ... Nazma N. Inamdar
