Affiliation:
Institute of Biochemistry, School of Nutritional Sciences, Food Science and Nutrition, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot 76100, Israel
Email: Diana.abuhalaka@mail.huji.ac.il
ORCID: https://orcid.org/0009-0003-1031-0088
Affiliation:
Institute of Biochemistry, School of Nutritional Sciences, Food Science and Nutrition, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot 76100, Israel
ORCID: https://orcid.org/0009-0009-1610-7818
Affiliation:
Institute of Biochemistry, School of Nutritional Sciences, Food Science and Nutrition, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot 76100, Israel
ORCID: https://orcid.org/0009-0000-8147-984X
Affiliation:
Institute of Biochemistry, School of Nutritional Sciences, Food Science and Nutrition, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot 76100, Israel
ORCID: https://orcid.org/0000-0001-5407-0440
Explor Dig Dis. 2025;4:100581 DOl: https://doi.org/10.37349/edd.2025.100581
Received: December 19, 2024 Accepted: June 26, 2025 Published: July 09, 2025
Academic Editor: Jose J. G. Marin, University of Salamanca, Spain
The article belongs to the special issue Gastrointestinal Diseases, Cholesterol, Oxysterols, and Bile Acids
Aim: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a widespread chronic liver condition associated with liver inflammation, fibrosis, and various metabolic disorders. Although cholic acid (CA), a primary bile acid (BA), is known to reduce steatosis when added to a high-fat diet, it may exacerbate hepatocellular injury by promoting oxidative stress and inflammation. Therefore, regulating BA-induced liver toxicity is crucial. Dimethyl fumarate (DMF) is an FDA-approved drug known to activate the nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2), a transcription factor that induces cytoprotective genes involved in cellular stress. The present study aimed to investigate whether DMF supplementation could attenuate CA-induced liver injury in high-fat diet-fed mice.
Methods: To induce liver injury, high-fat diet with and without CA were compared for liver damage and liver fat gain. Following the establishment of the toxic but antisteatotic effect of CA, C57BL/6j mice were fed a high-fat diet supplemented with 0.5% CA (HFDCA) with or without DMF (0.3 mg/mL or 0.6 mg/mL), which was administered via drinking water for 7 weeks.
Results: CA was found to be an accelerator of high-fat diet to induce liver damage, but prevented liver fat accumulation. HFDCA mice showed signs of liver damage, including elevated liver enzymes and liver enlargement. However, DMF treatment activated the Nrf2 pathway and partially mitigated the hepatotoxic effect of CA/high-fat diet, although some doses exhibited pro-oxidant effects.
Conclusions: The findings suggest that DMF, as an activator of Nrf2, has potential as a therapeutic agent for liver diseases related to high-fat diets and BA-induced injury, though careful dosage management is crucial to maximize its benefits and mitigate BA toxicity.
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a common chronic liver condition characterized by excessive fat accumulation in hepatocytes alongside metabolic impairment and affecting more than 30% of people worldwide [1–3]. MASLD includes a spectrum of liver conditions ranging from simple steatosis to a more severe condition of metabolic dysfunction-associated steatohepatitis (MASH) characterized by liver inflammation and fibrosis, which may progress to cirrhosis [4].
Bile acids are amphipathic molecules generated in the liver from cholesterol by the classic or alternative pathways. They play an essential role in the digestion and absorption of dietary lipids and fat-soluble vitamins [5]. Given their crucial role in lipid metabolism, bile acids have been explored as therapeutic agents for MASLD and MASH, as they serve as endogenous ligands for the farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (GPBAR1, also known as TGR5) [6, 7]. Pharmacological activation of FXR has been shown to enhance metabolic balance and reduce liver inflammation in experimental MASLD models. Obeticholic acid (OCA), for instance, an FXR agonist, has demonstrated the ability to reduce liver fibrosis, enhance insulin sensitivity, and mitigate hepatic steatosis. FXR activation suppresses de novo lipogenesis by suppressing sterol regulatory element-binding protein 1 (SREBP-1) and ChREBP [8, 9] while promoting fatty acid oxidation through PPARα and FGF21 activation [10, 11]. Additionally, it attenuated NF-κB-mediated inflammation [12].
Dietary habits significantly influence bile acid metabolism. The Western diet, characterized by high red and processed meat consumption, alters bile acid homeostasis due to its high saturated fat and cholesterol content. Notably, patients with steatohepatitis exhibit elevated hepatic levels of bile acid, including chenodeoxycholic acid (CDCA), cholic acid, and deoxycholic acid (DCA) [13]. Disruption in bile acid homeostasis can lead to hepatocellular damage, mitochondrial deterioration, and oxidative stress, ultimately triggering an inflammatory response that can progress to liver fibrosis, cirrhosis, and eventually liver failure [6, 14, 15]. Thus, while bile acids have the potential to be used as therapeutic agents, their toxic effects at high concentrations must be considered for careful handling.
The transcription factor nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) plays a pivotal role in electrophiles detoxification and metabolic homeostasis, including the regulation of bile acid enterohepatic circulation and bile secretion [16, 17]. In normal conditions, the levels of Nrf2 protein in the cytoplasm are kept low by its inhibitory protein Keap1 (Kelch-like ECH-associated protein 1), which promotes the degradation of Nrf2 through ubiquitin conjugation and acts as a sensor of cellular stress [18, 19]. Upon exposure to electrophiles, Keap1 undergoes covalent modifications, releasing Nrf2 and allowing it to accumulate in the nucleus. In the nucleus, Nrf2 binds to the antioxidant response element (ARE), driving the expression of cytoprotective genes involved in detoxification [20–22]. Notably, Nrf2 activation has been shown to alleviate lipotoxicity and inflammation, yet its potential to counteract bile acid-induced liver injury remains largely unexplored. One of the most effective activators for Nrf2 is dimethyl fumarate (DMF), which is used as an oral drug for multiple sclerosis [23–25]. DMF was recently approved for its capacity to activate the Nrf2 transcription factor while suppressing the SREBP-1c protein levels in high-fat diet mice, hence reducing hepatic fat accumulation. Also, the anti-inflammatory activities of DMF have been established [26].
This study aims to determine whether activating the Nrf2/Keap1/ARE pathway by DMF can mitigate the hepatotoxic effect of the cholic acid in a high-fat diet, thus providing new insights into the potential of Nrf2-targeted therapies for enhancing bile acid safety in MASLD treatment.
Cholic acid from bovine or ovine bile was purchased from MP (MP BIO, 81-25-4). DMF, Aldrich-242926 was purchased from Sigma-Aldrich (USA). DMF was dissolved in alkaline mineral water (pH ≈ 8.2) to form solutions of 0.3 mg/mL and 0.6 mg/mL.
Six- to seven-weeks-old male C57BL/6J mice (n = 24) were obtained from the Authority for Biological and Biomedical Models (Jerusalem, Israel) and randomly divided into three groups (n = 8): (1) normal diet (control) AIN-93G [27], 16% fat from calories (ND); (2) high-fat diet with 60% fat (HFD); and (3) high-fat diet with 60% fat and 0.5% (w/w) cholic acid (HFDCA). Mice were fed ad libitum with their respective diets and had free access to water for six weeks under controlled conditions (12-hour light/dark cycle, 22 ± 2°C). Body weight and food intake were recorded weekly.
In this treatment study, six- to seven-weeks-old male C57BL/6J mice (n = 40) were randomly assigned to five experimental groups (n = 8): (1) normal diet (control) AIN-93G, 16% fat from calories (ND); (2) normal diet with 0.6 mg/mL DMF in water (ND + 0.6 mg/mL); (3) high-fat diet with 60% fat and 0.5% (w/w) cholic acid (HFDCA); (4) HFDCA with 0.3 mg/mL DMF (HFDCA + 0.3 mg/mL DMF); and (5) HFDCA with 0.6 mg/mL DMF (HFDCA + 0.6 mg/mL DMF). Diet compositions are provided in Table S1. Mice were maintained under the same housing conditions, with free access to food and water for seven weeks. Body weight, food intake, and water consumption were recorded weekly.
At the end of both experiments, mice were fasted overnight (12 hours) and then anesthetized in a dedicated narcotic chamber with isoflurane vapor (Piramal Critical Care Inc., Bethlehem, PA, USA) until they consistently lost the righting reflex and no longer responded to pain stimuli. Immediately after removal from the chamber, mice were sacrificed by cervical dislocation, followed by bilateral thoracotomy prior to organ and tissue collection. Liver and adipose tissue were collected and weighed, with a portion of liver tissue fixed in 4% formaldehyde for histology and the remaining stored at −80°C for future analysis.
Blood was collected from the inferior vena cava at the end of the experiment. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, and triglycerides were measured by American Medical Laboratories (AML), Herzliya, Israel. Fasting glucose levels were measured in the tail-tip blood using a handheld Optimum Xceed glucometer (Abbott Diagnostic Care Ltd, Oxon, UK).
Histological slides were prepared by the Authority for Biological and Biomedical Models (Jerusalem, Israel). Liver tissues were embedded in paraffin, and serial sections (3–5 μm thick) were cut from each block and stained with hematoxylin and eosin (H&E). Immunohistochemical staining was performed on 4 µm formalin-fixed and paraffin-embedded sections using the Leica Bond MAX system (Leica Biosystems Newcastle Ltd, UK). Slides were baked at 60°C, dewaxed, and pretreated with epitope-retrieval solution (ER1, Leica Biosystems Newcastle Ltd, UK), followed by incubation for 30 minutes with either F4/80 antibody (1:200 70076 by Cell-Signaling) or cleaved-Caspase3 antibody (1:1,200 9661 by Cell-Signaling). Detection performed using the Leica Bond Polymer Refine HRP kit (Leica Biosystems Newcastle Ltd, UK). All slides were counter-stained with hematoxylin. Immunohistochemical staining performed by Gavish Research Services, Ness Ziona. All stained slides were photographed using the EVOS® FL Auto Imaging System. The hepatic lipid droplet counts were calculated using ImageJ software (ver 1.54, National Institutes of Health, Bethesda, Maryland, USA) based on ×20 magnification slides, analyzing three images per mouse and averaging the results from five mice per treatment.
Hepatic lipid content was quantified using the Folch method. A 50 mg tissue sample was homogenized in chloroform/methanol in a ratio of 2:1 (v/v) and centrifuged at 15,000 rpm for 15 minutes. The upper aqueous phase was discarded, and the lower organic phase was transferred to a pre-weighed, clean tube. The samples were evaporated to complete dryness, and the lipid fraction was weighed and normalized to the initial tissue sample weight.
Liver total RNA was isolated using Tri-Reagent (Sigma-Aldrich, Rehovot, Israel) according to the manufacturer’s protocol. Complementary DNA was prepared using a high-capacity cDNA Reverse Transcription Kit (Quanta BioSciences, Gaithersburg, MD, USA). Quantitative real-time PCR (RT-qPCR) was performed with a QuantStudio™ 1 Real-Time PCR instrument (Applied Biosystems, Singapore) with specific primers (listed in Table S2). Quantitative changes in gene expression were determined by normalizing to 18S mRNA.
For tissue collection, the same part of the liver was immediately collected, rinsed with cold PBS, snap-frozen in liquid nitrogen, and kept at –80°C. For metabolites extraction, frozen tissue weighing between 30 ± 2 mg was added to CK14 homogenizing tubes containing 1.4 mm ceramic beads (Bertin Corp, P000926-LYSK0-A) which were prefilled with 1,000 µL of cold (–20°C) metabolite extraction solvent consisting of methanol (Merck, 106035), acetonitrile (Merck, 100029), and water at a ratio of 5:3:2. Samples were homogenized at 4°C in a Precellys 24 Tissue Homogenizer (Bertin Corp, P002391-P24T0-A.0). Homogenization conditions were set to three cycles, 30 seconds each, at 6,500 rpm with a 30-second gap between each of the cycles to preserve low temperature. Homogenates were centrifuged at 18,000 g for 15 minutes at 4°C. The supernatant was then collected in microcentrifuge tubes and centrifuged under the same conditions. The cleared supernatants were transferred to glass HPLC vials (Agilent, 8010-0542) and kept at –80°C until LC-MS analysis (Supplementary methods). The remaining methods and Table S3 are included in the Supplementary material.
Results are presented as mean ± SEM unless otherwise stated in the figure legend. Data were analyzed by the JMP 16 Pro software suites (SAS Institute, Cary, NC, USA). Comparisons between groups were made by one-way analysis of variance (ANOVA), followed by a post hoc Tukey-Kramer HSD test. Differences were considered significant at probability levels of p < 0.05 and indicated by different letters.
To determine the effect of cholic acid in a high-fat diet model, we conducted a 6-week experiment in which mice were randomly assigned to one of three diet groups: ND, HFD, or HFDCA. Following the 6-week feeding, livers and epididymal white adipose tissue (eWAT) were collected and weighed.
Table 1 presents body and tissue weights. As expected, mice on the high-fat diet exhibited higher final body weight compared to those on a normal diet. However, HFDCA tended to reduce final body weight compared to the HFD mice (p = 0.073).
The effect of dietary cholic acid in high-fat diet model on body parameters
| Parameters | ND | HFD | HFDCA |
|---|---|---|---|
| Initial body weight (g) | 19.23 ± 0.48 | 19.56 ± 0.66 | 19.56 ± 0.32 |
| Final body weight (g) | 24.87 ± 0.90b | 30.12 ± 1.59a | 26.37 ± 0.73ab |
| eWAT weight (g) | 0.63 ± 0.07b | 1.32 ± 0.16a | 0.89 ± 0.06b |
| eWAT: body weight (%) | 2.74 ± 0.24b | 4.94 ± 0.44a | 3.52 ± 0.21b |
| Liver weight (g) | 0.87 ± 0.03b | 0.86 ± 0.03b | 1.13 ± 0.02a |
| Liver: body weight (%) | 3.82 ± 0.06b | 3.29 ± 0.06c | 4.51 ± 0.12a |
Mice were fed for 6 weeks. Treatment groups: normal diet (ND), high-fat diet with 60% fat (HFD), high-fat diet with 60% fat and 0.5% (w/w) cholic acid (HFDCA). All values are expressed as mean ± SEM (n = 8). Values marked with different letters (a, b, c) are significantly different (p < 0.05) in the Tukey-Kramer post hoc test. eWAT: epididymal white adipose tissue
HFD-fed mice had larger eWAT weights compared to ND and HFDCA groups. Additionally, the eWAT-to-body weight ratio was significantly higher in the HFD group than in the other groups (p < 0.05). Similarly, liver weights were assessed. While no significant difference was observed between the ND and HFD groups, the HFDCA group had significantly higher liver weights compared to both (p < 0.0001). Furthermore, the HFD group had a lower liver-to-body weight ratio than the ND and HFDCA groups, with the HFDCA group having the highest ratio (p < 0.001).
To evaluate metabolic parameters, we measured fasting blood glucose, serum triglycerides, and total and HDL cholesterol levels. HFD-fed mice exhibited significantly higher fasting glucose levels compared to the ND group. Additionally, the HFDCA mice showed even further increased glucose levels (p < 0.01; Figure 1A). Serum triglyceride levels were comparable between the ND and HFD groups, whereas the HFDCA group showed a significant reduction relative to both (Figure 1B). Total cholesterol levels were significantly elevated in the HFD and HFDCA groups compared to the ND group (Figure 1C). However, HDL cholesterol levels were similar between the HFDCA and ND groups, while higher in the HFD group (Figure 1D).
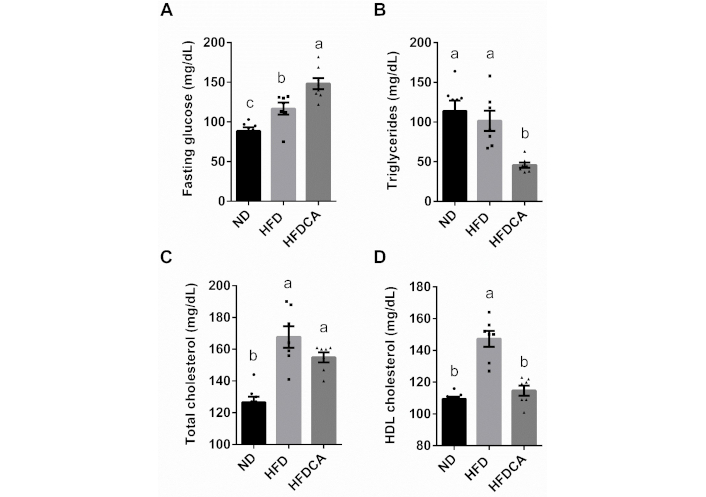
The effect of dietary cholic acid in a high-fat diet model on serum fasting glucose and lipid profile. (A) Fasting glucose; (B) total triglycerides levels; (C) total cholesterol; and (D) HDL cholesterol levels. Treatment groups: normal diet (ND), high-fat diet with 60% fat (HFD), high-fat diet with 60% fat and 0.5% (w/w) cholic acid (HFDCA). All values are expressed as mean ± SEM (n = 7 or 8) and each individual measurement represented by point. Columns marked with different letters (a, b, c) are significantly different (p < 0.05) in the Tukey-Kramer post hoc test
Elevated levels of serum AST and ALT levels are indicative of liver injury and hepatocytes death. The HFDCA group exhibited significantly higher AST and ALT levels compared to both the ND and the HFD groups (p < 0.0001). In contrast, no significant differences in liver enzyme levels were observed between the ND and HFD groups (Figure 2A, B). To further investigate liver fat accumulation, hepatic lipid content was quantified using the Folch lipid extraction method. HFD-fed mice tended to show higher liver lipid levels compared to the ND group (p = 0.057), while HFDCA reduced hepatic lipid accumulation relative to the HFD group (Figure 2C). Histological analysis of liver sections stained with H&E revealed that HFD-fed mice exhibited both microvascular and macrovascular steatosis, characterized by substantial hepatic lipid accumulation, as evidenced by the presence of lipid vacuoles (Figure 2D, E). In contrast, livers from ND- and HFDCA-fed mice showed minimal or no evidence of steatosis, suggesting that cholic acid supplementation exerts an anti-steatogenic effect against diet-induced hepatic fat accumulation.
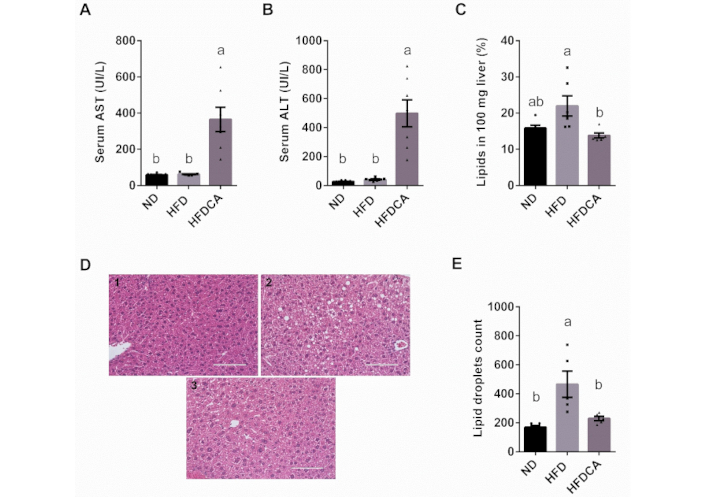
The effect of dietary cholic acid in a high-fat diet model on liver enzymes and H&E liver staining. Mice were fed for 6 weeks. Treatment groups: normal diet (ND), high-fat diet with 60% fat (HFD), high-fat diet with 60% fat and 0.5% (w/w) cholic acid (HFDCA). (A) Serum aspartate aminotransferase (AST) levels; (B) serum alanine aminotransferase (ALT) levels; (C) evaluation of liver lipid content using the Folch method; (D) H&E staining of liver sections under 40× magnification using digital microscope the EVOS® FL Auto Imaging System (scale bar, 100 μm); and (E) morphological analysis of liver droplet counts using ImageJ software of (1) ND; (2) HFD, and (3) HFDCA. Lipid droplets were counted from ×20 magnification slides with an area of 621,378 µm². All values are expressed as mean ± SEM (n = 5–8) and each individual measurement represented by point. Columns marked with different letters (a, b) are significantly different (p < 0.05) in the Tukey-Kramer post hoc test
Although hepatic lipid accumulation and liver enzyme levels varied across the groups, hepatic malondialdehyde (MDA) levels, a marker of oxidative stress, did not differ significantly among the groups (data not shown).
In the bile acid/DMF treatment study, mice were fed either a normal diet (ND) or a high-fat diet with 0.5% (w/w) cholic acid (HFDCA) and treated with DMF in drinking water for 7 weeks. Body weights, tissues and intake measurements are presented in Table 2. As expected, given the anti-obesogenic properties of cholic acid, final body weight did not differ significantly between the ND and HFDCA groups. Surprisingly, DMF supplementation in both dietary conditions (ND + 0.6 mg/mL DMF, HFDCA + 0.3 mg/mL or 0.6 mg/mL DMF) resulted in a lower final body weight compared to the corresponding non-DMF-supplemented groups (ND and HFDCA).
Effect of DMF supplementation on body parameters
| Parameters | ND | ND + 0.6 mg/mL DMF | HFDCA | HFDCA + 0.3 mg/mL DMF | HFDCA + 0.6 mg/mL DMF |
|---|---|---|---|---|---|
| Initial body weight (g) | 19.17 ± 0.32 | 19.22 ± 0.23 | 18.88 ± 0.39 | 18.63 ± 0.42 | 18.88 ± 0.29 |
| Final body weight (g) | 28.54 ± 0.65a | 25.61 ± 0.58bc | 26.73 ± 0.83ab | 23.71± 0.59c | 23.31 ± 0.88c |
| eWAT weight (g) | 0.67 ± 0.06a | 0.55 ± 0.05a | 0.52 ± 0.07a | 0.28 ± 0.02b | 0.30 ± 0.04b |
| eWAT: body weight (%) | 2.51 ± 0.19a | 2.26 ± 0.15a | 2.05 ± 0.22a | 1.26 ± 0.08b | 1.30 ± 0.16b |
| Liver weight (g) | 1.07 ± 0.04ab | 0.93 ± 0.03c | 1.14 ± 0.04a | 1.00 ± 0.04bc | 1.07 ± 0.02ab |
| Liver: body weight (%) | 3.99 ± 0.08b | 3.82 ± 0.06b | 4.55 ± 0.15a | 4.55 ± 0.08a | 4.86 ± 0.12a |
| Calories intake (kcal/mouse/day) | 12.9 ± 1.38b | 10.8 ± 1.02b | 27.7 ± 9.43a | 17.6 ± 5.89b | 16.8 ± 4.58b |
| Calories intake (kcal/g body weight/day) | 0.55 ± 0.05b | 0.49 ± 0.03b | 1.28 ± 0.17a | 0.88 ± 0.14ab | 0.84 ± 0.11ab |
| Water consumption (mL/day) | 6.21 ± 0.52a | 4.15 ± 1.31b | 5.77 ± 0.88ab | 4.15 ± 0.87b | 3.98 ± 0.62b |
Male C57BL/6J mice aged 6–7 weeks were fed normal diet (ND), ND + 0.6 mg/mL dimethyl fumarate (DMF) by water, high-fat diet + 0.5% cholic acid (HFDCA), HFDCA + 0.3 mg/mL DMF by water, and HFDCA + 0.6 mg/mL DMF by water for 7 weeks. All values are expressed as mean ± SEM (n = 8). Values marked with different letters (a, b, c) are significantly different (p < 0.05) in the Tukey-Kramer post hoc test. eWAT: epididymal white adipose tissue
The decrease in body weight observed in the HFDCA group supplemented with either 0.3 or 0.6 mg/mL DMF was associated with reduced calorie intake when compared to the HFDCA group without DMF supplementation. Notably, when normalized to body weight, the calorie intake of the HFDCA group is higher than the ND groups and only the HFDCA + 0.6 mg/mL DMF mice tended to have reduced normalized calorie intake compared to the HFDCA (p = 0.078). However, no such difference in calorie intake was observed among the normal diet groups. The mice’s water consumption remained within the normal range throughout the trial.
Furthermore, liver and eWAT tissues were collected and weighed. While eWAT weights were comparable among the ND, ND + 0.6 mg/mL DMF, and HFDCA groups, mice supplemented with 0.3 or 0.6 mg/mL DMF in the HFDCA diet exhibited significantly lower eWAT. This pattern was similarly reflected in the eWAT-to-body weight ratio.
Regarding the liver, ND + 0.6 mg/mL DMF mice exhibited lower liver weight compared to the ND mice, although no difference in the liver-to-body weight ratio. In contrast, the HFDCA group had liver weights comparable to the ND group. Still, it showed a higher liver-to-body weight ratio. Among the CA-treated groups, mice supplemented with 0.3 mg/mL DMF had lower liver weight compared to the HFDCA group alone, yet the liver-to-body weight ratio did not differ significantly among the HFDCA, HFDCA + 0.3 mg/mL DMF, and HFDCA + 0.6 mg/mL DMF treatments.
The fasting glucose and serum lipid levels are shown in Figure 3. Mice fed the HFDCA diet exhibited significantly higher fasting blood glucose levels than both the ND and the ND group supplemented with 0.6 mg/mL DMF (p < 0.01). However, supplementation with 0.3 mg/mL DMF in the HFDCA diet significantly reduced glucose levels compared to the HFDCA group without DMF (p = 0.001; Figure 3A). Additionally, total triglyceride levels were significantly lower in all HFDCA-fed groups than in the ND and ND + 0.6 mg/mL groups. In contrast, total cholesterol levels remained unchanged across the experimental groups (Figure 3B, C).
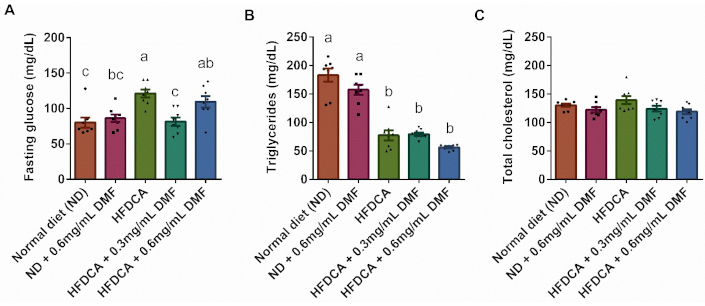
Serum fasting glucose and lipid profile parameters. (A) Fasting glucose; (B) total triglycerides; and (C) total cholesterol levels. All values are expressed as mean ± SEM (n = 8) and each measurement is represented by point. Columns marked with different letters (a, b, c) are significantly different (p < 0.05) in the Tukey-Kramer post hoc test
Liver sections from mice were stained with H&E. As shown in Figure 4, the liver samples exhibited well-defined hepatocyte structure and nuclei, no apparent signs of steatosis, reinforcing the protective antisteatotic effect of cholic acid with or without DMF. However, closer examination of the HFDCA group revealed morphologic changes in hepatocytes, which belong to small cell change of dysplasia (SCD), characterized by open nucleus and increased nuclear to cytoplasmic ratio. Also, some areas revealed malformed and shrunken nuclei, as well as intralobular spotty necrosis marked by the loss of some hepatocytes and disintegrating nucleus, indicating the onset of tissue damage and liver pathology. Despite these histological changes, liver damage was not related to inflammation. F4/80 immunostaining, used to assess macrophage infiltration, showed minimal or no differences between the treatments. Additionally, no significant changes in inflammatory gene expression were detected (Figure S1). Given the antisteatotic effect of cholic acid, the mRNA levels of genes involved in cholesterol and bile acid metabolism, including CYP7A1 (cholesterol-7α-hydroxylase), FXR, and SHP (small heterodimer partner) were analyzed. The analysis revealed significantly reduced mRNA levels of CYP7A1, a rate-limiting enzyme in bile acid synthesis, in all HFDCA-fed mice (HFDCA, HFDCA + 0.3 or 0.6 mg/mL DMF) compared to the ND. However, the mRNA levels of SHP and FXR were unaffected by the treatments (Figure S2).
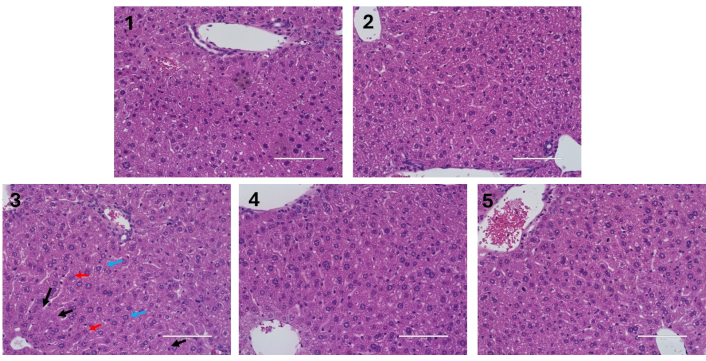
Liver histology. Hematoxylin and eosin (H&E) staining of liver sections under ×40 magnification using digital microscope, the EVOS® FL Auto Imaging System (scale bar, 100 μm). Black arrows indicate the absence or deletion of hepatocytes, red arrows indicate for disintegrating nucleus, and blue arrows indicate small cell change (dysplasia). Male C57BL/6J mice aged 6–7 weeks were fed (1) normal diet (ND); (2) ND + 0.6 mg/mL DMF by water; (3) high-fat diet + 0.5% cholic acid (HFDCA); (4) HFDCA + 0.3 mg/mL DMF by water; and (5) HFDCA + 0.6 mg/mL DMF by water for 7 weeks
Liver injury markers, including liver enzymes, oxidative stress indicators, and apoptotic markers, were assessed. DMF supplementation in the normal diet group had no hepatotoxic effect, as AST and ALT levels were comparable to those in the ND group. As anticipated, the HFDCA group showed significantly elevated AST and ALT levels compared to both the ND and ND + 0.6 mg/mL DMF groups (p < 0.0005; Figure 5A, B). Similarly, the HFDCA + 0.3 mg/mL DMF group also exhibited higher AST and ALT levels than the two normal diet groups. Notably, ALT levels were significantly lower in the HFDCA + 0.6 mg/mL DMF group compared to the HFDCA group (p = 0.0098), while AST levels showed no significant difference. Of note, bilirubin levels remained consistent across all groups (data not shown).
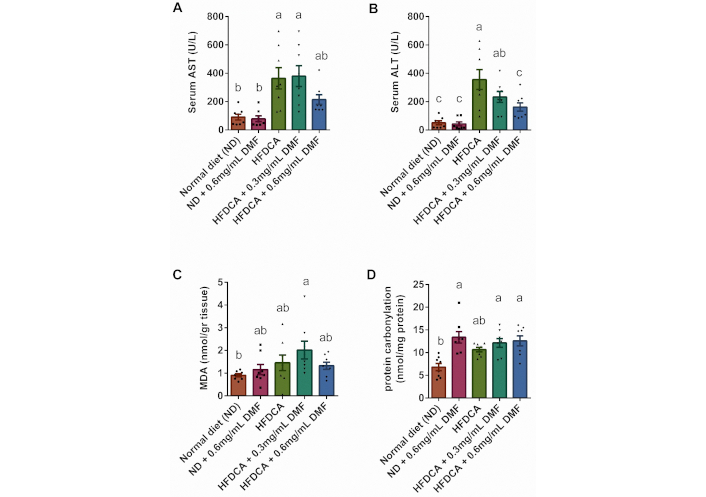
Serum liver enzymes and oxidative stress. Serum (A) AST and (B) ALT levels; (C) malondialdehyde (MDA) levels; and (D) protein carbonylation. All values are expressed as mean ± SEM (n = 7 or 8) and each individual measurement represented by point. Columns marked with different letters (a, b, c) are significantly different (p < 0.05) in the Tukey-Kramer post hoc test. AST: aspartate aminotransferase; ALT: alanine aminotransferase
To further evaluate liver tissue integrity and potential fibrotic changes. Masson’s Trichrome staining was performed. No notable differences in collagen deposition were observed between the groups, suggesting that HFDCA consumption for 7 weeks, regardless of DMF supplementation, had not yet promoted fibrosis (Figure S3).
To assess the extent of oxidative stress, MDA levels and protein carbonylation, were measured. MDA levels did not differ significantly between the ND and HFDCA groups. However, compared to the ND group, MDA levels were two-fold higher in the HFDCA + 0.3 mg/mL DMF group (Figure 5C). Regarding protein carbonylation, all three DMF-supplemented groups (ND + 0.6 mg/mL DMF, HFDCA + 0.3 mg/mL DMF and HFDCA + 0.6 mg/mL DMF) exhibited significantly higher levels of carbonylated protein. In contrast, the HFDCA group showed a trend toward elevated levels relative to the ND group (p = 0.0564; Figure 5D).
Caspase-3 activity and immunohistochemical staining, both key markers of apoptosis initiation in the liver, were assessed. No significant difference in caspase-3 activity was observed between the ND and ND + 0.6 mg/mL DMF groups. However, supplementation with 0.3 mg/mL DMF in the HFDCA diet significantly increased caspase-3 activity compared to ND group, suggesting the initiation of apoptosis within the liver tissue (Figure 6A). Three experimental groups were selected for immunohistochemical analysis of active caspase-3 staining, as they exhibited significant alteration in liver enzymes levels in serum. Representative immunohistochemical-staining for caspase-3 in ND, HFDCA, and HFDCA + 0.6 mg/mL DMF groups is shown in Figure 6B, with the HFDCA group displaying areas indicative of cleaved caspase-3. Moreover, assessment of the hepatic proliferative cell nuclear antigen (PCNA) protein levels, a marker for cell proliferation, revealed increased expression in the HFDCA group compared to the ND group (Figure 6C). This finding aligns with the elevated liver-to-body weight ratio observed in HFDCA-fed mice. Collectively, the increase in liver tissue weight, caspase-3 activity, and PCNA protein expression suggests compensatory proliferation in the liver.
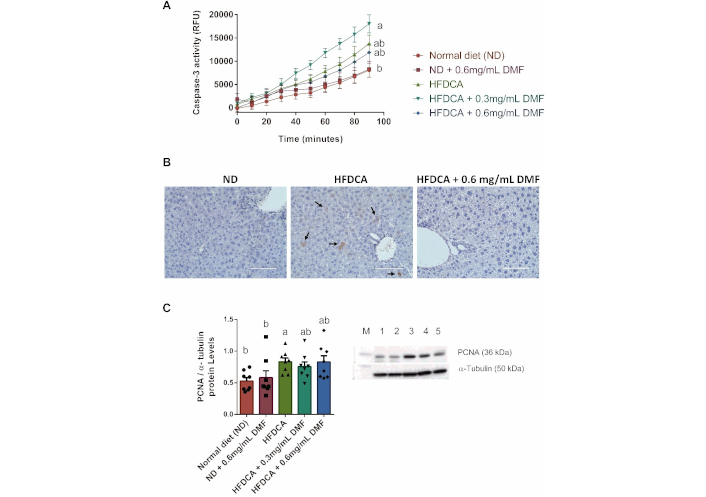
Hepatic apoptosis and regeneration. (A) Liver caspase-3 activity and (B) representative immunohistochemical staining of liver cleaved caspase-3 indicated by black arrows (brown), (×40 magnification; scale bar, 100 µm); and (C) representative western blot of PCNA protein and statistical results normalized by α-tubulin (Lane M: protein ladder, Lane 1–5: ND, ND + 0.6 mg/mL DMF, HFDCA, HFDCA + 0.3 mg/mL DMF, and HFDCA + 0.6 mg/mL DMF). All values are expressed as mean ± SEM (n = 8) and each measurement is represented by point. Curves and columns marked with different letters (a, b) are significantly different (p < 0.05) in the Tukey-Kramer post hoc test. PCNA: proliferative cell nuclear antigen
DMF was added to the diets in order to stimulate the Nrf2 transcription factor, thereby inducing the expression of genes associated with AREs. Since DMF is a known Nrf2 activator and is administered orally, Nrf2 nuclear translocation was not evaluated using a western blot method. Rather, we evaluated the hepatic expression of Nrf2-regulated genes and the levels of thiol-conjugates, which represent DMF metabolism and its effect on glutathione. These analyses ensure that DMF reaches the liver and activated Nrf2 as intended.
Accordingly, HFDCA + 0.6 mg/mL DMF mice had significantly elevated mRNA levels of glutathione S-transferase A1 (GSTA1), NAD(P)H quinone dehydrogenase 1 (NQO1), and glutamate-cysteine ligase catalytic subunit (GCLC) compared to normal diet (Figure 7B–D). However, in comparison between HFDCA and HFDCA + 0.6 mg/mL DMF, only the mRNA levels of NQO1 and GCLC were significantly higher among the HFDCA + 0.6 mg/mL DMF, with a trend toward an increase in the GSTA1 (p = 0.0524). Interestingly, there were no differences in the mRNA levels of the tested genes between the ND and ND + 0.6 mg/mL DMF groups. Also, none of the treatments influenced hemeoxygenase-1 (HO-1) mRNA expression (Figure 7A).
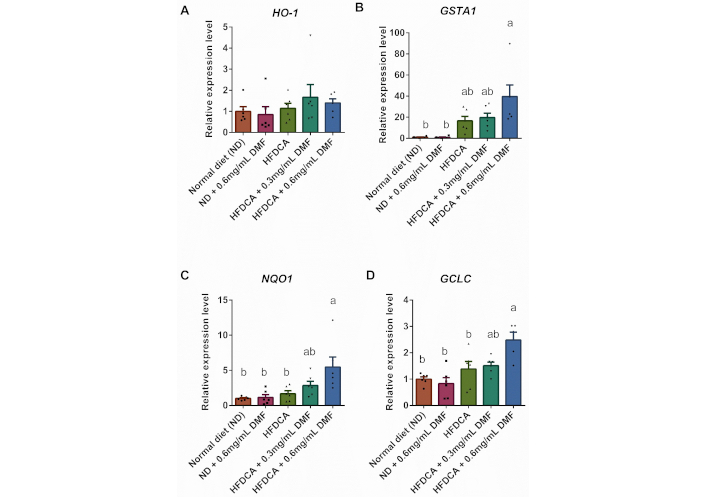
Hepatic expression of Nrf2-related genes. mRNA levels of (A) hemeoxygenase-1 (HO-1), (B) glutathione S-transferase A1 (GSTA1), (C) NAD(P)H quinone dehydrogenase 1 (NQO1), and (D) glutamate-cysteine ligase catalytic subunit (GCLC). All values are expressed as mean ± SEM (n = 6) and each measurement is represented by point. Columns marked with different letters (a, b) are significantly different (p < 0.05) in the Tukey-Kramer post hoc test
Since HFDCA + 0.6 mg/mL DMF mice exhibited increased hepatic levels of Nrf2-related genes mRNA expression, specifically GSTA1 and GCLC—both are involved in glutathione synthesis and conjugation—liver samples were analyzed via metabolomics to further confirm the effect of DMF. This analysis identified 241 compounds, with Principal Component Analysis (PCA) and heatmap analysis revealing distinct metabolic profiles, across the different groups. Notably, the metabolic profiles of liver samples from mice on ND and HFDCA differed significantly, regardless of DMF supplementation (Figure S4).
As expected, DMF supplementation did not alter fumarate levels, as DMF primarily undergoes hydrolysis to form its active metabolite, monomethyl fumarate (MMF), rather than being directly converted to fumarate. Additionally, cysteine and cystine levels remained unchanged (Figure S5), and the reduced-to-oxidized glutathione (GSH/GSSG) ratio, as well as hepatic thiol levels, were consistent across all groups (data not shown).
However, analysis of DMF-glutathione conjugates revealed a significant increase in DMF metabolites (MMF, fumarate) conjugated with cysteine and N-acetylcysteine in both the ND + 0.6 mg/mL DMF and the HFDCA + 0.6 mg/mL DMF compared to their respective non-supplemented mice (ND and HFDCA) (Figure 8A–D). The IUPAC names of these fumarate-glutathione conjugates are provided in Table S4. These findings highlight the strong capacity of DMF to interact with thiols in the liver to activate defense pathways.
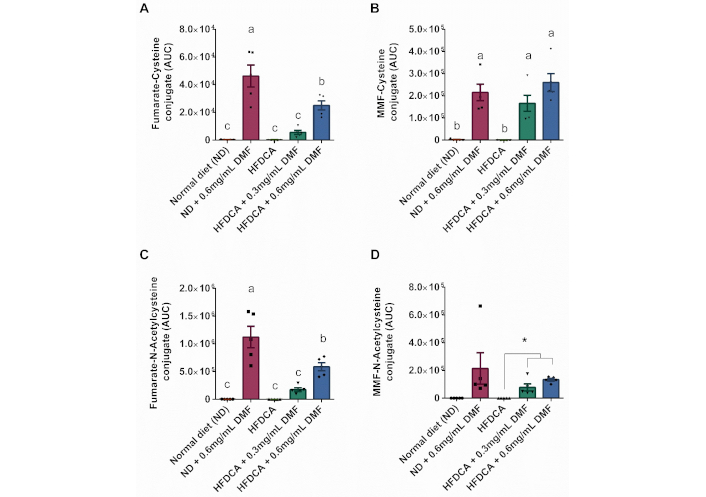
Hepatic DMF-glutathione conjugates levels. (A–D) LC-MS analysis of the indicated metabolites. All values are expressed as mean ± SEM (n = 5) and each measurement is represented by point. Columns marked with different letters (a, b, c) are significantly different (p < 0.05) in Tukey-Kramer post hoc test, and columns connected with asterisks are statistically different according to t-test (* indicates p < 0.01)
This study investigated the role of cholic acid in a high-fat diet and assessed the protective effects of DMF, an Nrf2 activator, against bile-acid-induced liver injury. We demonstrated that 0.5% (w/w) cholic acid supplementation to the high-fat diet has an anti-obesogenic effect, indicated by body weight and decreased eWAT-to-body weight ratio. Additionally, hepatic lipid accumulation was markedly reduced in HFDCA-fed mice, highlighting the potential of cholic acid to alleviate steatosis. However, cholic acid supplementation also resulted in elevated serum levels of the liver enzymes AST and ALT, as well as an increased liver-to-body weight ratio, indicating hepatotoxicity. However, oral DMF treatment attenuated liver injury, as evidenced by reduced ALT levels. Furthermore, DMF supplementation in HFDCA-fed mice improved body and metabolic parameters, including lower final body weight, decreased eWAT-to-body weight ratio, and normalized calorie intake which result in improved anti-obesogenic effect of cholic acid.
Bile acids are amphipathic molecules that are essential for the absorption of dietary lipids and fat-soluble vitamins [28]. Beyond their classical role, bile acids also act as signaling molecules involved in the regulation of the homeostasis of lipids, glucose, and energy by activating various receptors such as FXR and GPBAR1 [29]. Several bile-acid-based therapies, particularly ursodeoxycholic acid (UDCA) and CDCA, have been tested in clinical trials for liver diseases, including cholestatic liver disease and primary biliary cirrhosis [30–32]. Also, targeting FXR receptors with bile acids, their derivatives, or bile acid conjugates has been proposed as a potential therapeutic strategy for metabolic diseases, including MASLD. In line with this, Watanabe et al. (2004) [33] demonstrated that dietary cholic acid supplementation reduced hepatic triglyceride accumulation in mice with hypertriglyceridemia. Similarly, Cipriani et al. (2010) [9] reported that activating FXR with a synthetic cholic acid derivative, 6-ethyl-chenodeoxycholic acid (6E-CDCA or OCA) improved insulin resistance and decreased hepatic steatosis in Zucker fa/fa rats. We showed that supplementing a high-fat diet with CDCArg (chenodeoxycholyl-arginine ethyl ester conjugate) reduced liver steatosis, upregulated PPARα, and downregulated SREBP1 expression. These were accompanied by reductions in body weight, testicular fat, and liver tissue weight [34].
On the other hand, hydrophobic bile acids such as glycocholic acid (GCA), lithocholic acid (LCA), cholic acid, CDCA, and DCA, can induce hepatocyte cell death by disrupting membranes or triggering apoptosis [35, 36]. Studies in hepatocytes have shown that intracellular bile acid accumulation can lead to Fas receptor clustering and activate the TNF-related apoptosis-inducing ligand receptor, resulting in caspase-8 activation [37, 38].
In our study, supplementation of 0.5% (w/w) cholic acid to high-fat diet—based on previously investigated concentration (0.5% or 1% w/w) used in atherogenic diet to enhance cholesterol absorption and induce metabolic disturbances [39, 40]—resulted in final body weights in HFDCA-fed mice that were comparable to those of normal diet-fed mice. This outcome aligns with findings by Watanabe et al. (2006) [41], who showed that cholic acid supplementation increased metabolic rate, as evidenced by elevated CO2 production and O2 consumption. Although these measurements were not acquired in our work, our findings suggest a similar mechanism, possibly involving the activation of energy expenditure pathways. This assumption is further supported by the consistent observation of higher food consumption in the presence of cholic acid, as seen in our previous study [34]. As a result, cholic acid feeding amplified the high-fat diet-induced reduction in the respiratory quotient (RQ), indicating increased fat oxidation. Furthermore, the total serum triglyceride levels in fasting mice fed the HFDCA were lower than in normal and high-fat diet, indicating the beneficial effects of the cholic acid supplementation, as a high carbohydrate diet can promote hypertriglyceridemia [42].
Importantly, mice fed HFDCA exhibited no signs of hepatic steatosis compared to the high-fat diet-fed mice. The anti-steatotic effect of cholic acid may be attributed to the reduction in CYP7A1 mRNA expression, potentially indicating FXR activation, which enhances fat oxidation and reduces lipid accumulation [43].
However, cholic acid supplementation led to elevated liver enzymes, including AST and ALT, as well as an increased liver-to-body weight ratio. These findings are consistent with Song et al. (2011) [44], who demonstrated that hepatotoxicity can occur even at cholic acid concentrations as low as 0.3% in the feed. Despite that, mice fed a high-fat diet supplemented with cholic acid had normal bilirubin levels, suggesting that the injury may predominantly affect hepatocytes rather than cholangiocytes, or that it represents an early stage of liver damage. HFDCA-fed mice also displayed an increased liver-to-body weight ratio, accompanied by elevated PCNA protein expression and proliferation process. PCNA regulates the cell cycle and is essential for liver regeneration [45, 46]. PCNA can modulate the levels of p21 by either promoting its degradation or binding and activating it, affecting the cell’s decision to repair or to undergo apoptosis [47]. In response to DNA damage, the tumor suppressor p53 is activated, causing the buildup of p21 and inducing cell-cycle arrest to allow DNA repair. Apoptosis is triggered when the damage is irreversible [48]. Cholic acid supplementation may induce proliferation in the liver for increased liver size. This is in line with our previous study, which demonstrated that dietary supplementation with cholic acid and cholesterol—even in the absence of excess dietary fat—resulted in marked hepatocyte proliferation and significantly increased liver weight [49]. Thus, the primary anti-obesogenic effect of cholic acid appears to be mediated through a reduction in adipose tissue mass.
Treatment with DMF, an activator for Nrf2, resulted in improvement in both liver health and overall body metabolism. Oral administration of DMF led to reduced final body weight, eWAT-to-body weight ratio, and normalized calorie intake compared to the HFDCA-fed mice, highlighting the role of DMF in enhancing metabolism and regulating food intake in high-fat-diet-fed mice. In an in vivo study, Shin et al. (2009) [50] demonstrated that oral administration of CDDO-Im, another potent Nrf2 activator, was associated with increased oxygen consumption and energy expenditure.
Notably, mice fed an HFDCA and treated with the higher, but not the lower, dose of DMF exhibited reduced ALT levels, suggesting that the higher DMF dose counteracts bile acid-induced liver injury. Liver damage was primarily evaluated by measuring liver enzyme levels, with further histological and immunohistochemical analyses. H&E staining revealed regions of hepatocyte loss and nuclear disintegration, indicative of small cell changes of dysplasia and necrosis. Additionally, immunohistochemical staining for cleaved caspase-3 showed positive staining, suggesting apoptotic activity. In contrast, F4/80 staining showed no macrophage infiltration, and Masson’s Trichrome staining revealed no significant collagen deposition at this stage. Taken together, these findings suggest an increased rate of apoptosis in the liver and the early stages of liver injury. Interestingly, treatment with the lower dose of DMF in the HFDCA group higher levels of hepatic MDA. This suggests that the lower DMF dose may not be beneficial and could even act as a pro-oxidant under certain conditions and dosage, causing harm to the tissue [51, 52].
DMF, as an activator of the Nrf2/Keap1/ARE pathway, provides an important defense mechanism against electrophiles without exerting direct antioxidant effects like polyphenols. A higher dose of DMF proved more effective in protecting against cholic acid-induced liver injury, accompanied by the upregulation of key Nrf2-related protective genes, including GSTA1, NQO1, and GCLC. However, HO-1 mRNA expression remained unchanged. This may be attributed to the complex regulation of HO-1, which is regulated not only by Nrf2 but also by other transcription factors such as hypoxia-inducible factor 1-alpha (HIF-1α), which may exert a stronger regulatory role under certain conditions [53]. DMF enhances Nrf2 activation by inducing covalent modifications of thiol groups in specific Keap1 cysteine residues, particularly cysteine 151. These modifications lead to structural changes that disrupt Keap1-Nrf2 interactions, facilitating Nrf2 translocation into the nucleus and promoting the expression of detoxification or antioxidant enzymes [54]. As demonstrated in our previous in vitro study, we observed Nrf2 translocation in response to DMF, supporting its role in activating this critical pathway [55]. Additionally, DMF depletes intracellular glutathione by forming DMF-glutathione adducts through a Michael addition reaction, resulting in a stabilization and increase of Nrf2 levels, ultimately enhancing glutathione turnover [56, 57]. In the present study, DMF-glutathione adducts were predominantly generated when a higher dose of DMF was supplemented in both the ND and the HFD with cholic acid (HFDCA).
Although our study does not demonstrate high oxidative stress conditions, it is evident that Nrf2 activation contributed to the reduction of blood liver enzymes, suggesting its role in mitigating liver injury. Nrf2, beyond its classical role in oxidative stress response, was also found to be crucial for the expression of hepatobiliary transporters and bile acid homeostasis. As shown by Weerachayaphoen et al. (2012) [17], Nrf2–/– mice exhibited a reduced biliary bile acid rate, elevated intrahepatic bile acids levels, and decreased expression of CYP7A1 and CYP8B1—key regulators of bile acid synthesis - compared to wild-type mice. Furthermore, the mRNA expression of bile acid transporters, including bile salt export pump (BSEP) and organic solute transporter (OSTα), was increased. These findings highlight the broader role of Nrf2 in maintaining bile acid balance, which may also help explain the observed effects of DMF on liver health.
This study provides a comprehensive analysis of both the beneficial and harmful effects of bile acid supplementation within in high-fat diet model, intended to mimic ingredients found in processed meat. Also, the use of two doses of DMF enabled us to observe dose-dependent effects and highlighted the potential role of low-dose DMF under certain conditions. However, our study has several limitations. First, the experiments were conducted at standard housing temperature (~22°C), rather than under thermoneutral conditions (~29°C), which more accurately reflect an optimal metabolic state of mice [58]. Since housing temperatures have an influence on energy metabolism and stress response, future studies should evaluate the effects of DMF under such conditions. Second, our study was conducted on male mice only, which limits the applicability of our findings to females. Further research involving both male and female is a particularly important consideration given the well-documented sex-specific differences in Nrf2 activation.
To conclude, the study highlights the therapeutic potential of DMF in mitigating bile acid-induced liver injury, particularly in a high-fat diet, through the activation of the Nrf2/Keap1/ARE pathway. We demonstrated that a high-fat diet supplemented with bile acid negatively impacts liver tissue, as indicated by elevated ALT and AST levels. By administering an appropriate dose of DMF, we observed its protective role in alleviating toxicity, improving steatosis, and moderating excess food consumption. Finally, these findings suggest that combining DMF alongside bile acid therapies may be worth investigating for managing steatotic liver disease, hence improving their efficacy. This combined approach offers potential in managing liver damage associated with high processed meat consumption.
ALT: alanine aminotransferase
ARE: antioxidant response element
AST: aspartate aminotransferase
CDCA: chenodeoxycholic acid
DCA: deoxycholic acid
DMF: dimethyl fumarate
eWAT: epididymal white adipose tissue
FXR: farnesoid X receptor
GPBAR1: G protein-coupled bile acid receptor 1
H&E: hematoxylin and eosin
Keap1: Kelch-like ECH-associated protein 1
MASH: metabolic dysfunction-associated steatohepatitis
MASLD: metabolic dysfunction-associated steatotic liver disease
MDA: malondialdehyde
MMF: monomethyl fumarate
Nrf2: nuclear factor erythroid 2 (NFE2)-related factor 2
OCA: obeticholic acid
PCNA: proliferative cell nuclear antigen
SREBP-1: sterol regulatory element-binding protein 1
The supplementary materials for this article are available at: https://www.explorationpub.com/uploads/Article/file/100581_sup_1.pdf.
The authors thank Dr. Adi Shpaizer for her part in the diet preparation and animals’ maintenance and Dr. Zohar Gavish at Gavish Research Services Ltd for performing the histological work.
DAH: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing—original draft, Writing—review & editing, Visualization. ER: Investigation, Formal analysis. HZ: Methodology, Formal analysis, Writing—review & editing. OT: Conceptualization, Methodology, Validation, Resources, Writing—original draft, Writing—review & editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Prof. Oren Tirosh is an Associate Editor and Guest Editor of Exploration of Digestive Diseases. However, he was not involved in any aspect of the peer review or editorial decision-making process for this manuscript. The other authors declare no conflicts of interest.
All animal procedures were performed following the guidelines of the Guide for the Care and Use of Laboratory Animals. The study was approved by the Animal Ethics Committee of the Faculty of Agriculture, Food and Environment, the Hebrew University of Jerusalem, approval number: AG-22-17063-3.
Not applicable.
Not applicable.
Data will be available upon request.
This work is funded by internal funding from the Hebrew University of Jerusalem. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1992
Download: 14
Times Cited: 0
