53 results in Exploration of Immunology
Latest
Sort by :
- Latest
- Most Viewed
- Most Downloaded
- Most Cited
Open Access
Original Article
Recombinant influenza A/H1N1pdm09 vaccine expressing streptococcal surface epitope for dual protection
Yulia Desheva ... Irina Isakova-Sivak
Published: November 28, 2025 Explor Immunol. 2025;5:1003230
This article belongs to the special issue Old and New Paradigms in Viral Vaccinology

Open Access
Review
HLA-KIRs interactions in modulating natural killer cell responses against viral hepatitis: a concise review
Ata Shirizadeh ... Ghasem Solgi
Published: November 23, 2025 Explor Immunol. 2025;5:1003229
This article belongs to the special issue Immunogenetics of Chronic Illnesses
Open Access
Systematic Review
Immune evasion mechanisms and cutting-edge therapeutic strategies of PD-L1 pathway in oral squamous cell carcinoma: an umbrella review
Neha Kannan ... Giuseppe Minervini
Published: November 17, 2025 Explor Immunol. 2025;5:1003228
This article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies
Open Access
Review
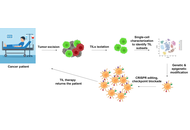
Probing and enhancing tumor-infiltrating lymphocytes: insights from single-cell technologies and genetic reprogramming
Chaitanya Kumar ... Veeraraghavan Vishnu Priya
Published: November 17, 2025 Explor Immunol. 2025;5:1003227
This article belongs to the special issue Advances and Novel Insights into Immunoinformatics
Open Access
Review
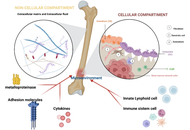
Phenotypic and functional alterations of innate lymphoid cells in hematological malignancies: potential clinical application
Mario Di Gioacchino ... Alessandro Allegra
Published: November 17, 2025 Explor Immunol. 2025;5:1003226
Open Access
Editorial
The discovery of Regulatory T Cells: a long journey toward immune balance and Nobel Prize
Anna Calabrò, Calogero Caruso
Published: November 06, 2025 Explor Immunol. 2025;5:1003225
Open Access
Review
Cancer immunotherapy and cardiovascular side-effects: from treatment modalities to the use of the preventive effect of antihypertensive drugs
Fakher Rahim ... Issenova Balday
Published: October 28, 2025 Explor Immunol. 2025;5:1003224
This article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies
Open Access
Review
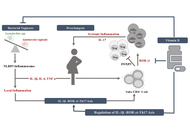
Translating the vaginal microbial landscape: a connecting link between bacterial vaginosis and preeclampsia
Devanshi Gajjar, Sriram Seshadri
Published: October 24, 2025 Explor Immunol. 2025;5:1003223
Open Access
Review
Cutaneous lupus erythematosus: insights from molecular pathogenesis to targeted therapies
Fatima K. Alduraibi ... Peter C. Chien
Published: October 24, 2025 Explor Immunol. 2025;5:1003222
Open Access
Case Report
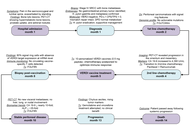
Personalized peptide vaccines induce predicted T cell responses against signet ring cell carcinoma—a case report
Julianna Lisziewicz ... Bartolome Garcia Perez
Published: October 13, 2025 Explor Immunol. 2025;5:1003221
Open Access
Review
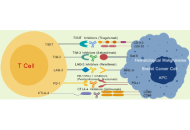
Role of immune checkpoint inhibitors in breast cancer and hematological malignancies
Qing Bao ... Hailin Tang
Published: September 29, 2025 Explor Immunol. 2025;5:1003220
This article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies
Open Access
Review
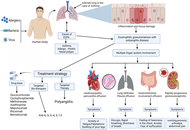
Eosinophilic granulomatosis with polyangiitis: overcoming diagnostic obstacles and exploring pharmacotherapeutic approaches
Priya Komre ... Debarshi Kar Mahapatra
Published: September 29, 2025 Explor Immunol. 2025;5:1003219
Open Access
Mini Review
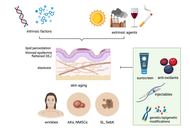
Skin aging and immunosenescence
Natasa Strbo ... Alessia Paganelli
Published: September 29, 2025 Explor Immunol. 2025;5:1003218
This article belongs to the special issue Immunosenescence: Mechanisms and Its Impact
Open Access
Original Article
Role of cyproheptadine in chronic urticaria not controlled with second-generation antihistamines: a retrospective study
Shambo S. Samajdar ... Shashank R. Joshi
Published: September 21, 2025 Explor Immunol. 2025;5:1003217
This article belongs to the special issue Hypersensitivity Syndrome Reactions versus Allergy and Drug

Open Access
Original Article
Crosstalk between autophagy and apoptosis in initiating antitumor immune responses in human lymphoma cells
Kayce Blumenstock ... Azizul Haque
Published: September 11, 2025 Explor Immunol. 2025;5:1003216

Open Access
Original Article
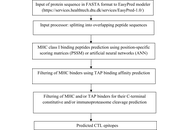
Forecast of cytotoxic T lymphocyte epitope using sequence weighting and artificial neural network based on EasyPred modeler
Satarudra Prakash Singh ... Bhartendu Nath Mishra
Published: September 09, 2025 Explor Immunol. 2025;5:1003215
Open Access
Original Article
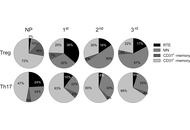
Features of peripheral blood Treg and Th17 subsets during physiological pregnancy
Olga Gorbunova ... Sergey Shirshev
Published: August 26, 2025 Explor Immunol. 2025;5:1003214
Open Access
Review
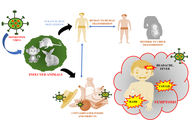
Understanding mpox pathogenesis: therapeutic potential of marine-derived drugs
Sourav Pal ... Khokan Bera
Published: August 26, 2025 Explor Immunol. 2025;5:1003213
Open Access
Review
Harnessing mRNA vaccines for viral diseases: bottleneck and breakthrough
Vijay Mishra ... Yachana Mishra
Published: August 22, 2025 Explor Immunol. 2025;5:1003212
This article belongs to the special issue Novel Vaccines development for Emerging, Acute, and Re-emerging Infectious Diseases
Open Access
Review
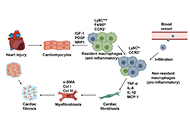
Macrophages and fibroblasts in cardiac fibrosis: interactions and transformation
Daehun Kim, Jea-Hyun Baek
Published: August 18, 2025 Explor Immunol. 2025;5:1003211