Representative pharmacotherapies currently under investigation for the treatment of MASLD/MASH
| Mechanism | Selected compounds | Structure | Remarks |
|---|---|---|---|
| FXR agonism | Obeticholic acid | 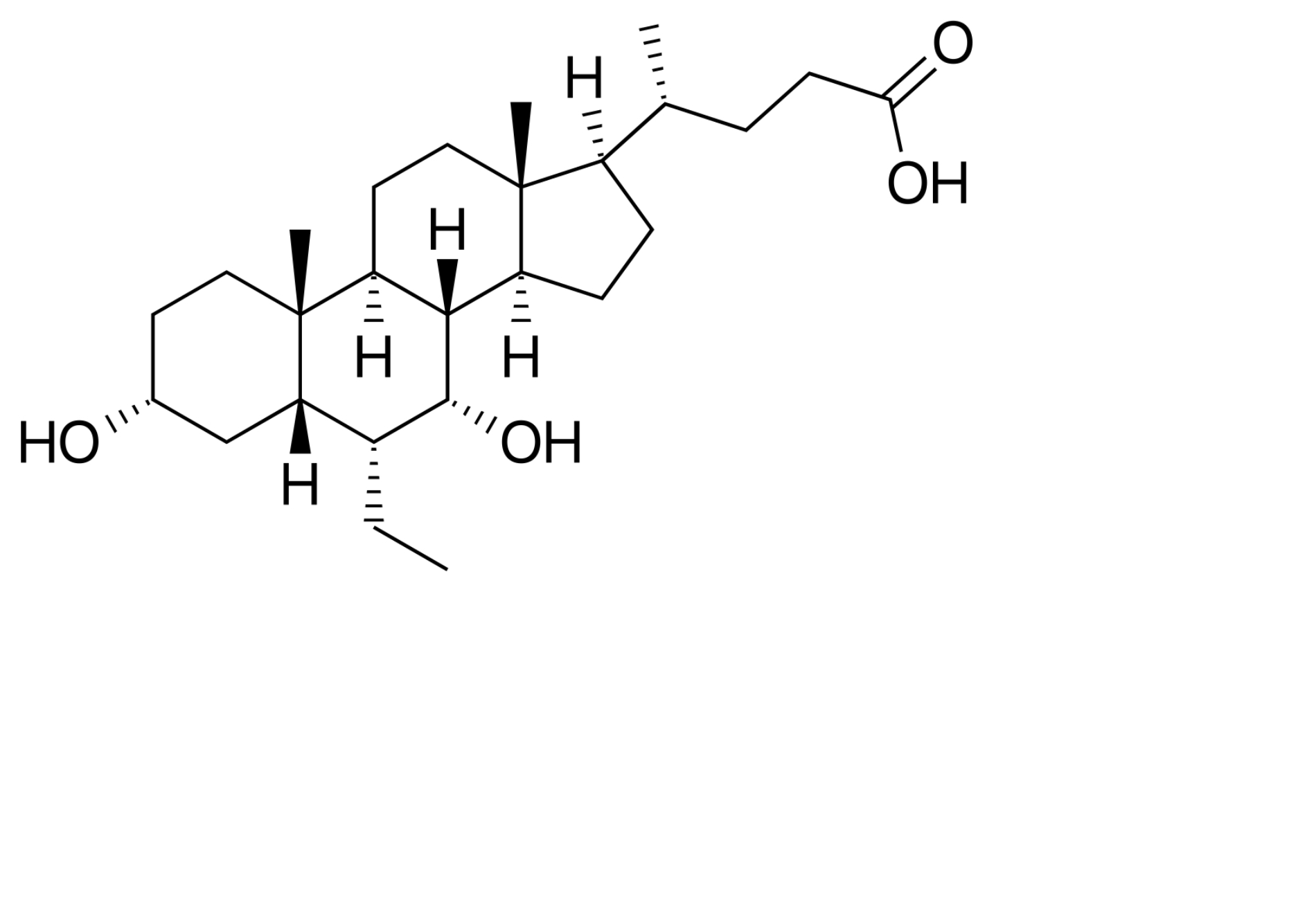 | Obeticholic acid is a semi-synthetic bile acid data that is FDA approved for primary biliary cholangitis. It ameliorates obesity and hepatic steatosis by activating brown fat [141]. |
| Tropifexor | 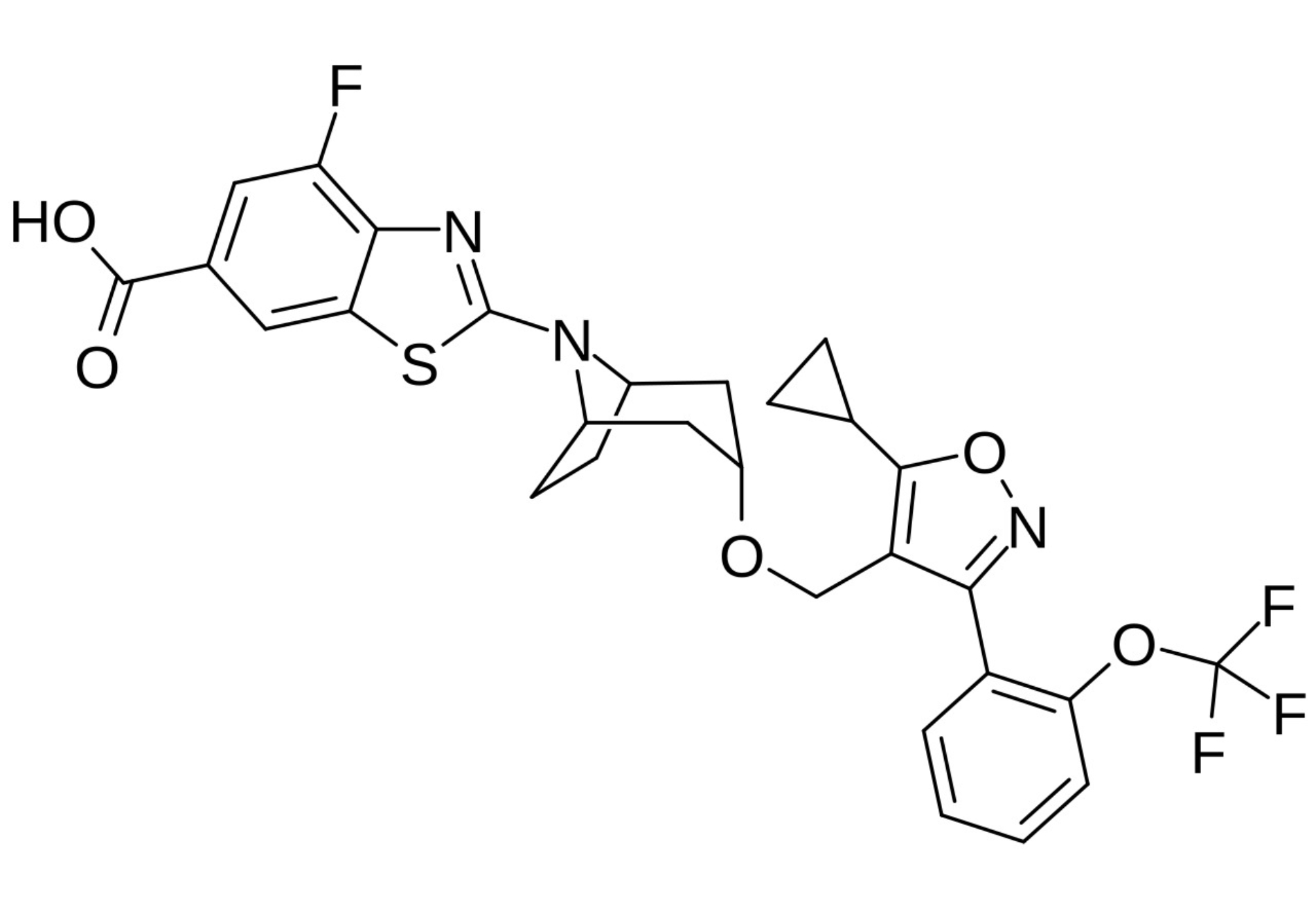 | Tropifexor was developed for the treatment of cholestatic liver diseases and MASH [142]. | |
| Cilofexor | 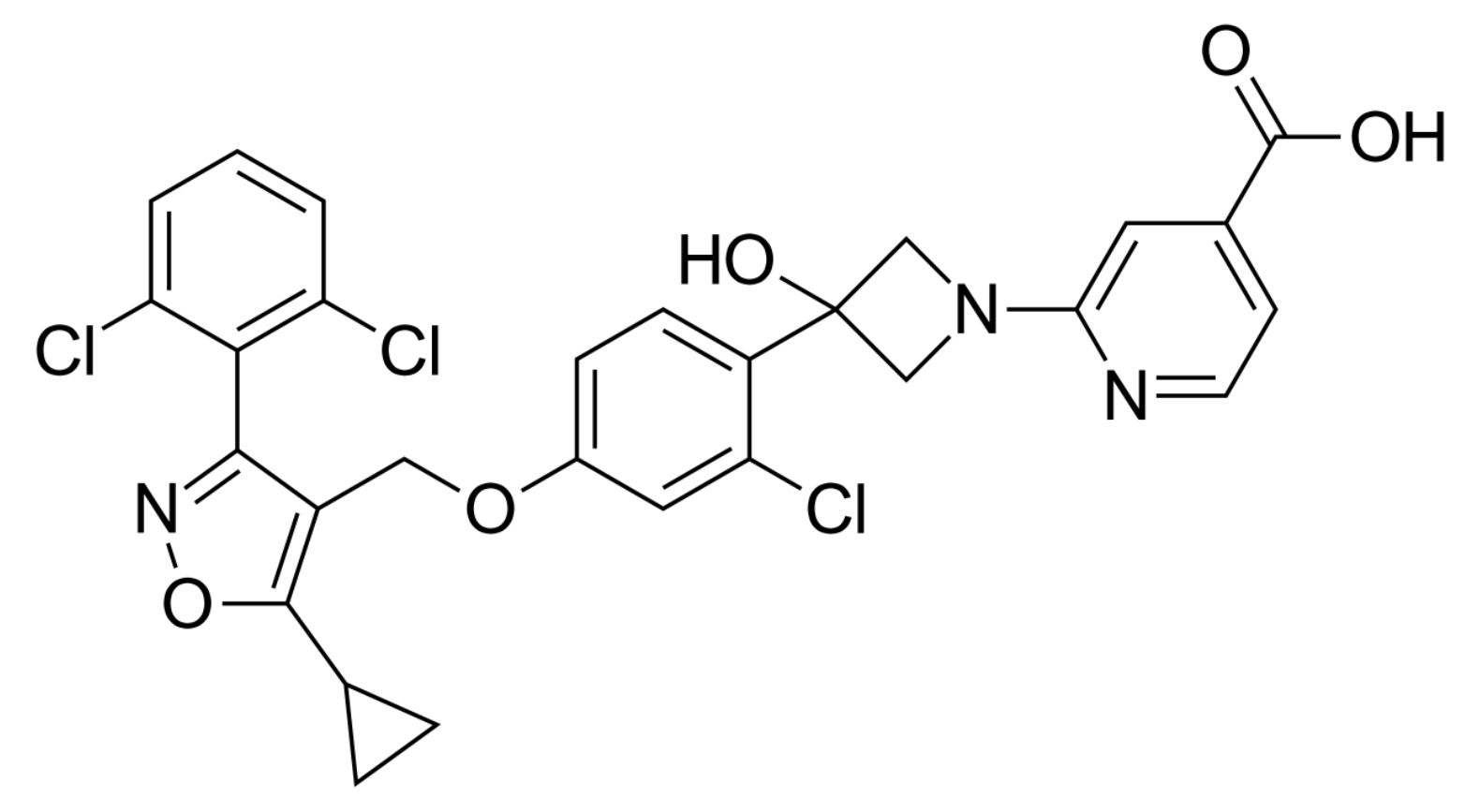 | Cilofexor is a nonsteroidal FXR agonist for the treatment of MASLD, MASH, and primary sclerosing cholangitis [143]. | |
| PPAR agonism | Elafibranor | 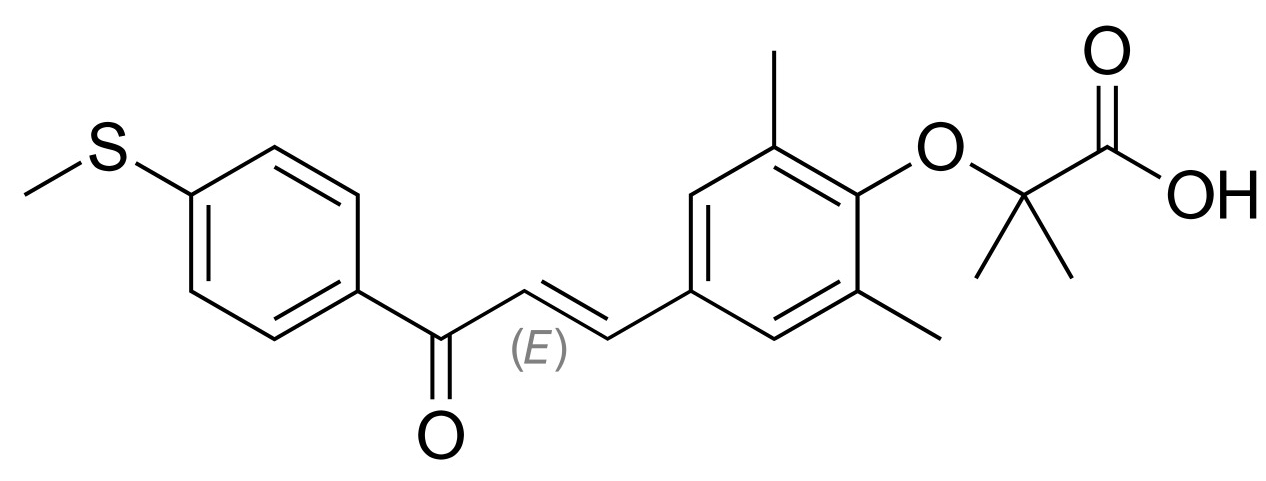 | Elafibranor is a dual PPAR α/δ agonist that is approved for the treatment of primary biliary cholangitis, showing beneficial effects in MASH [144]. |
| Saroglitazar | 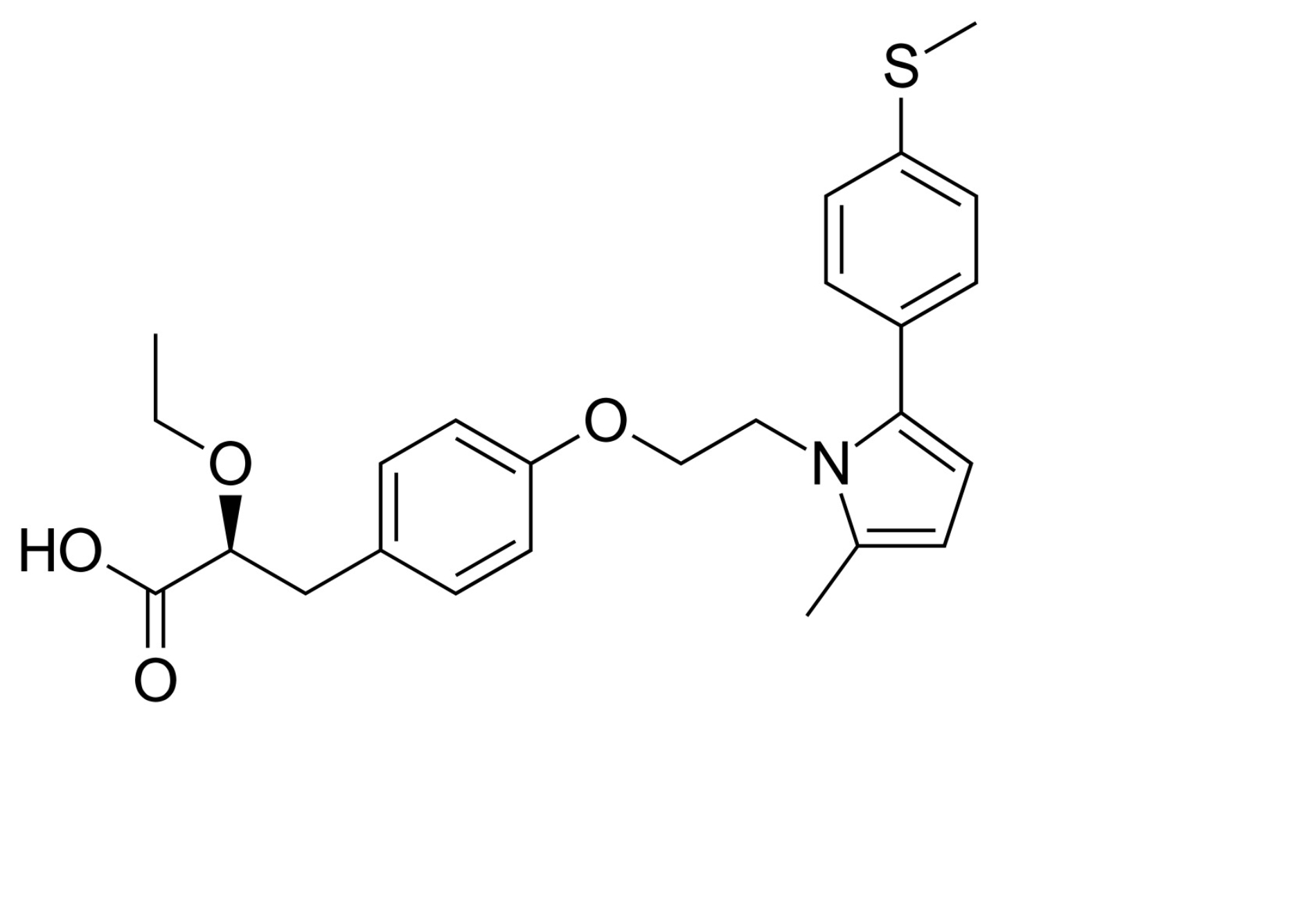 | Saraglitazar is a dual PPARα/γ agonist acting as an insulin sensitizer and has beneficial effects on adipose tissue and extracellular matrix deposition in obesity. Therefore, it is a drug for the treatment of T2DM, dyslipidemia, MASLD, and MASH [145]. | |
| Lanifibranor | 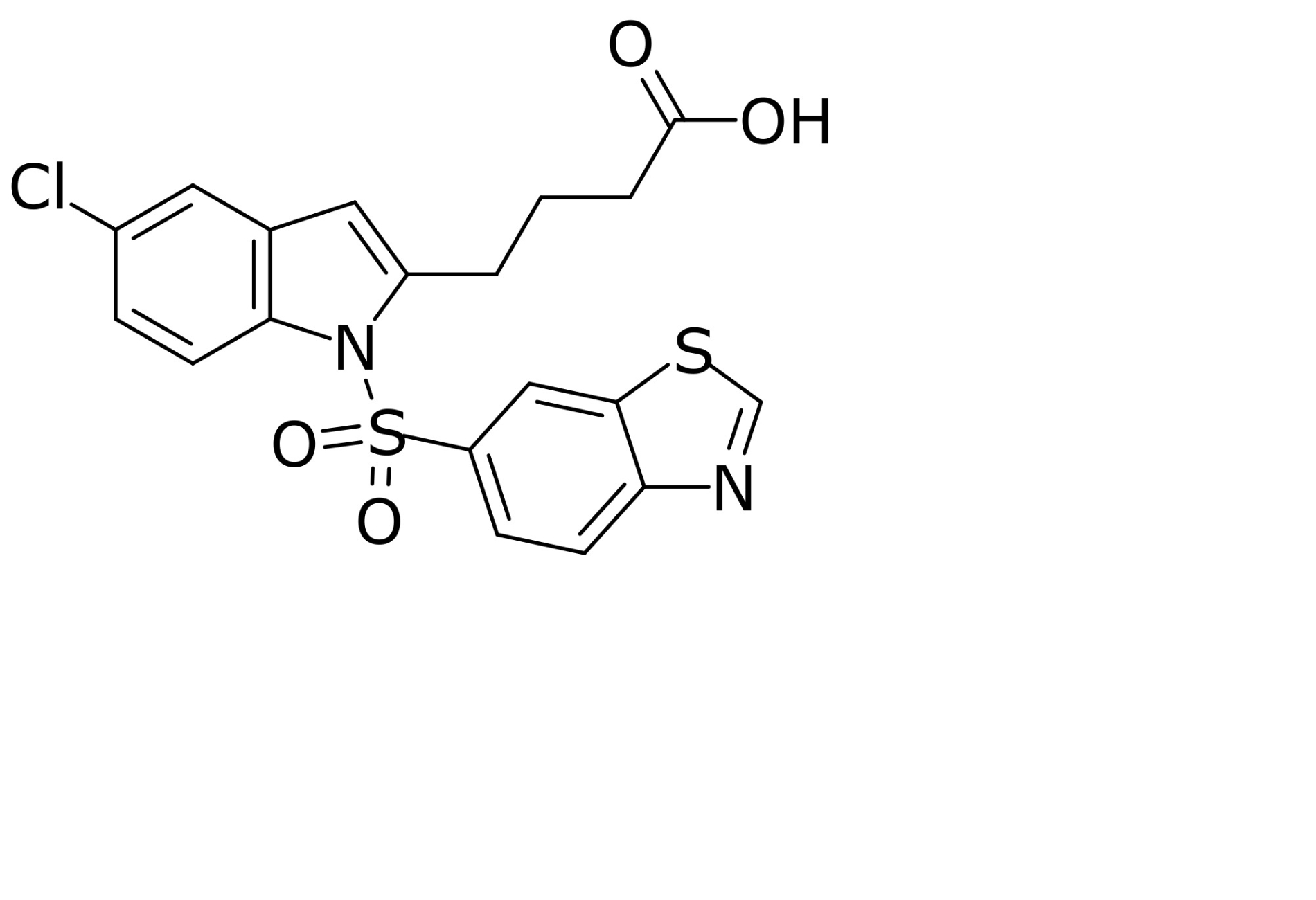 | Lanifibranor is a pan-specific PPAR agonist with balanced α, β/δ, and γ activity. It improves liver health, IR, adipose tissue function, and provokes weight gain in MASH patients [146, 147]. | |
| ACC inhibition | Firsocostat (GS-0976) | 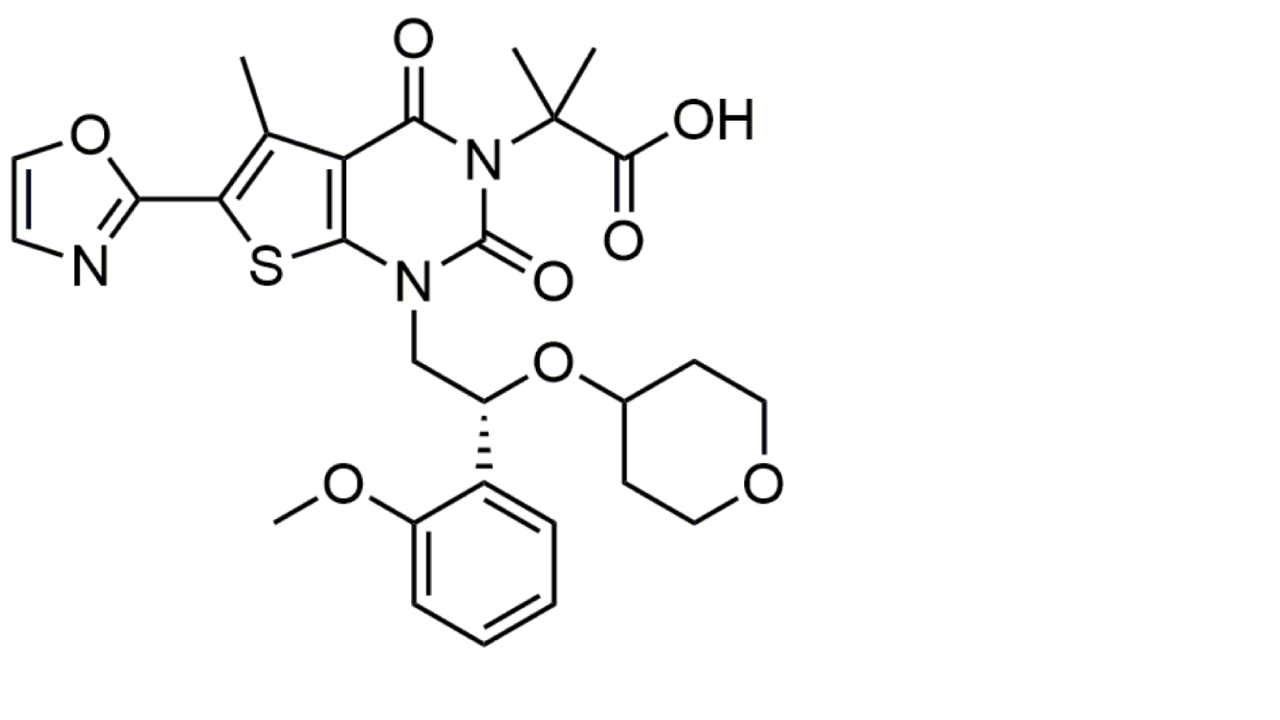 | Firsocostat is a liver-targeted ACC inhibitor that inhibits hepatic de novo lipogenesis and decreases the deleterious effects of lipotoxicity. It reduces hepatic steatosis that, in combination with other drugs, is therapeutically effective in MASH [148]. |
| PF-05221304 (Clesacostat) | 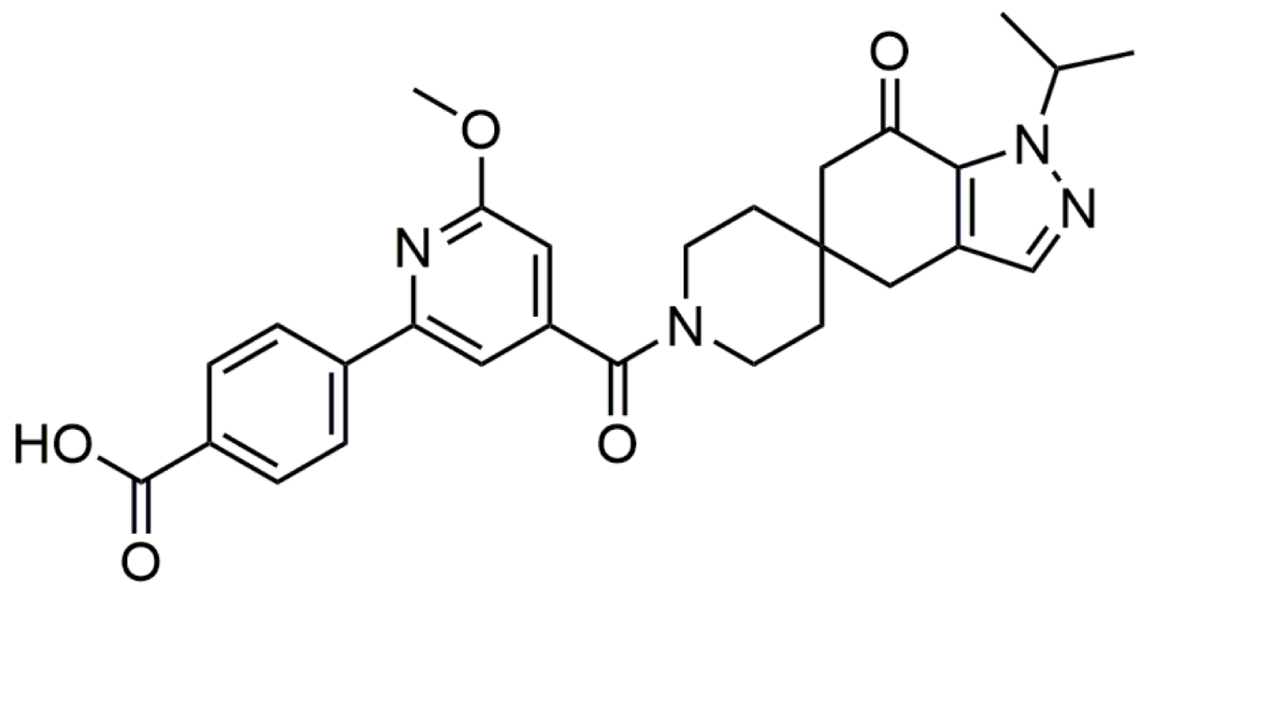 | PF-05221304 is an orally bioavailable, liver-targeted inhibitor of ACC. It inhibits de novo lipogenesis and is therefore under close investigation for treatment of MASLD/MASH [149]. | |
| THRβ agonism | Resmetirom | 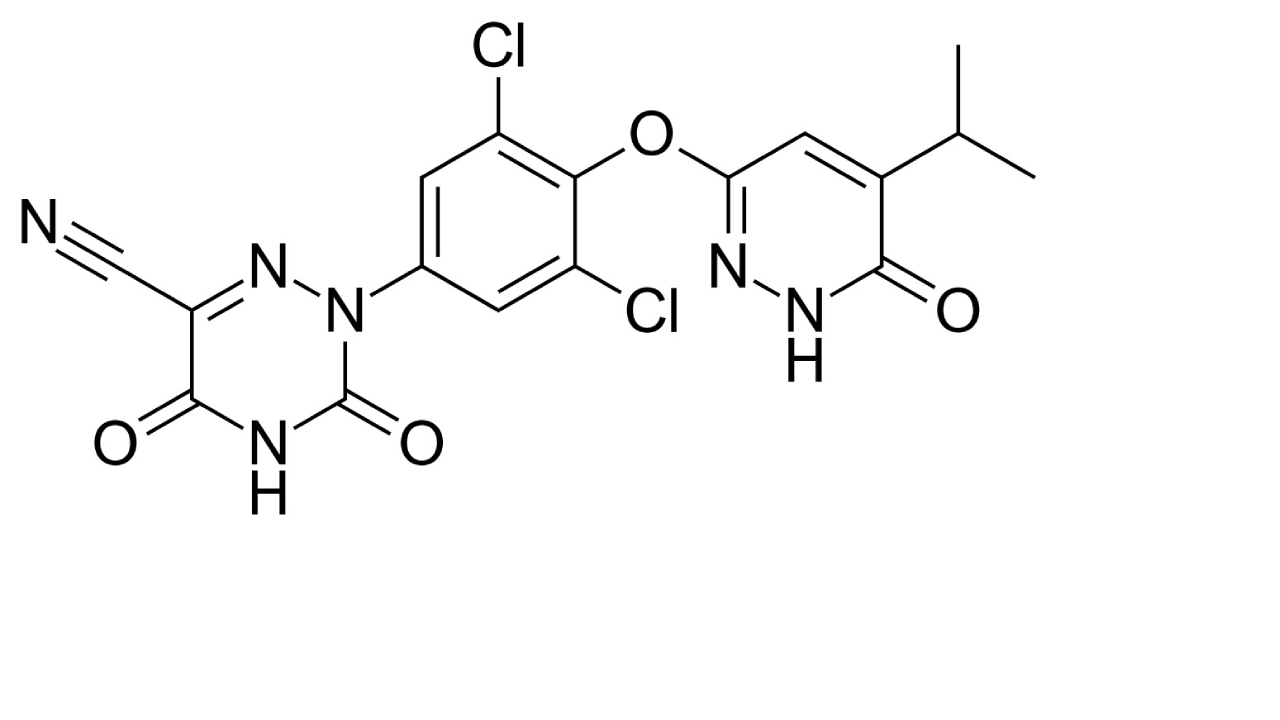 | Resmetirom is a FDA-approved drug used for treatment of non-cirrhotic MASH with moderate to advanced liver fibrosis [150]. |
| FGF17 mimetics | Aldafermin | 5-194 FGF19 (5-Methionine, 6-Arginine, 8-Serine, 9-Serine, 11-Leucine); calculated molecular weight of 21,300 Da. | Aldafermin is an engineered analog of the human hormone FGF19 that improves liver histology in patients with non-cirrhotic MASH and patients with compensated MASH cirrhosis [151]. |
| FGF21 mimetics | Pegbelfermin (BMS-986036) | (109-(4-(1-((2-((ω-Methoxypoly(oxyethylene))formamido)ethoxy)imino)ethyl)-L-Phenylalanine))homo sapiens FGF21; calculated average molecular of 50,000 Da. | Pegbelfermin is a polyethylene glycol-conjugated analog of human FGF21 that impacts energy metabolism and reduces hepatic fat fraction in patients with MASH [152, 153]. |
| SCD1 inhibition | Aramchol | 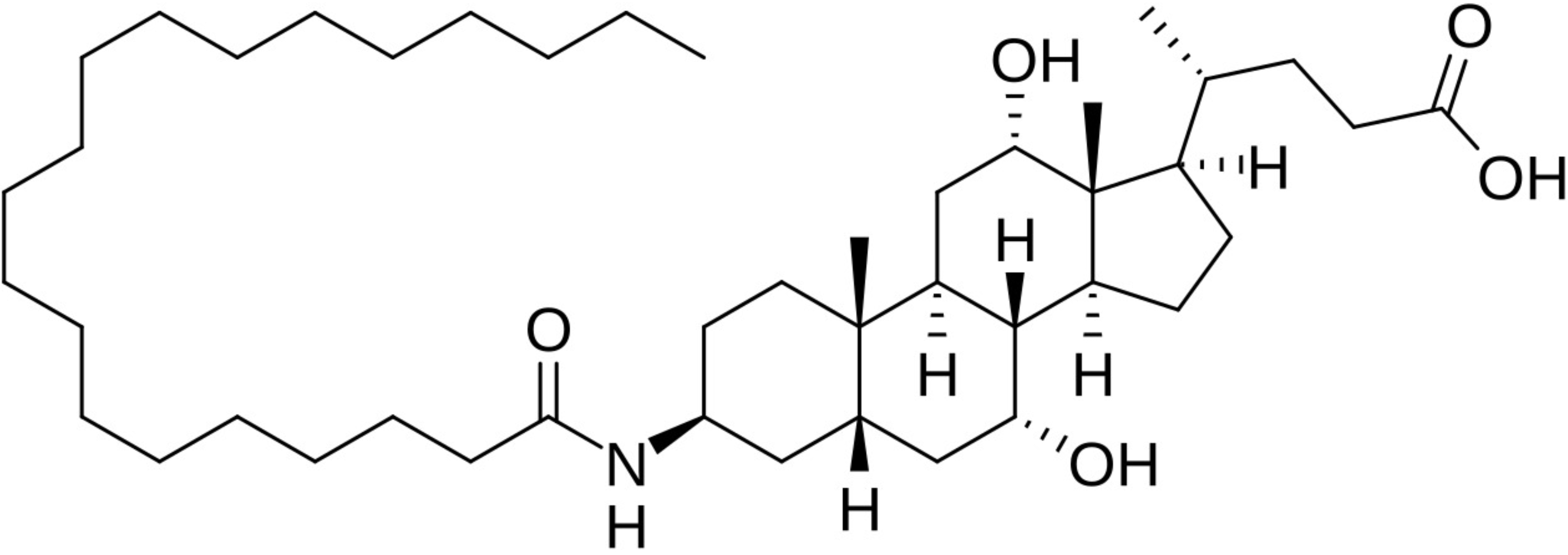 | Aramchol is a conjugate of cholic acid and arachidic acid that can be orally administered for treatment of MASLD/MASH. It affects liver fat metabolism and reduces liver fat content by inhibiting the activity of SCD1 in the liver [154]. |
| CCR2/CCR5 inhibition | Cenicriviroc | 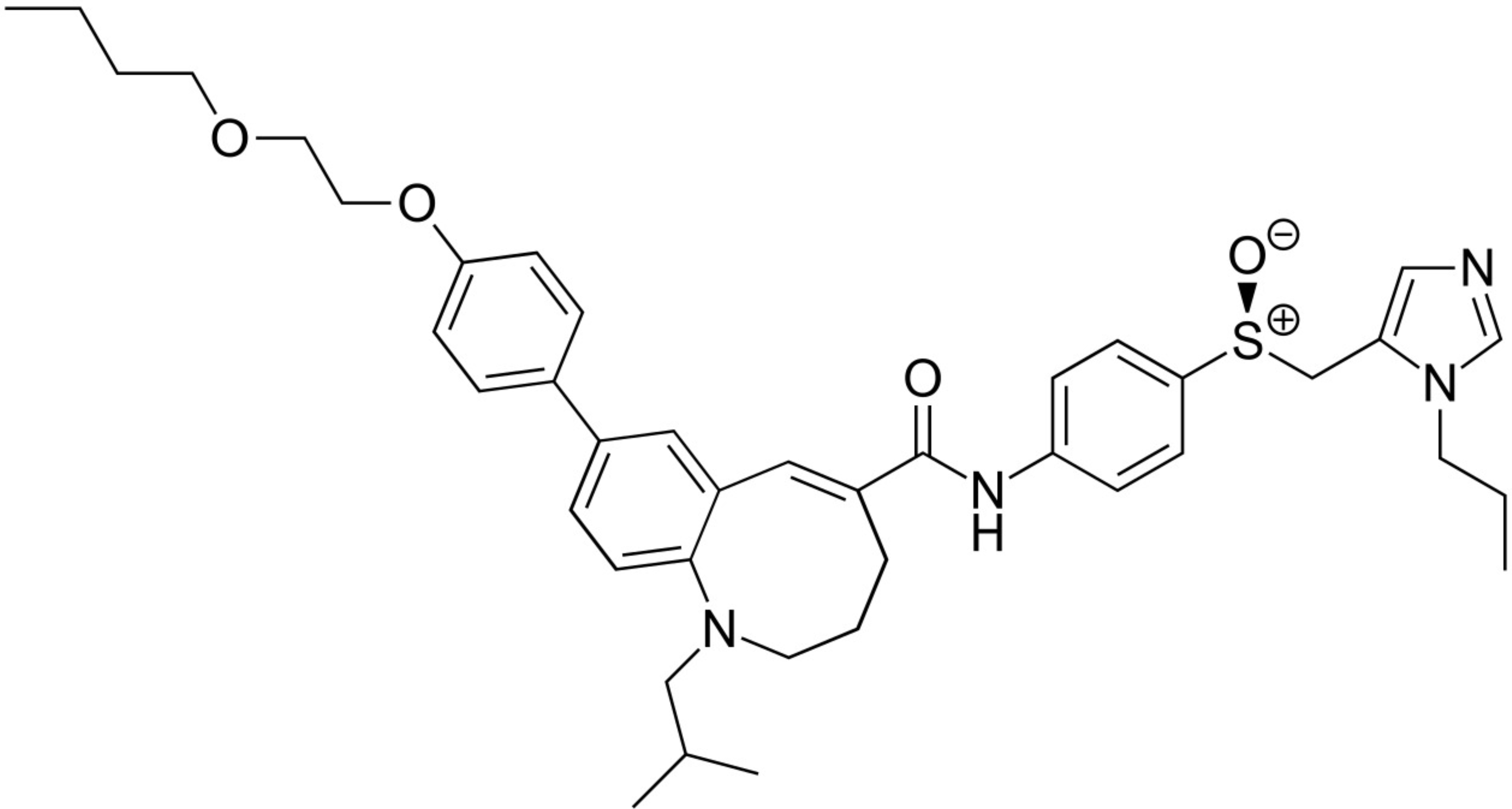 | Initially developed for the treatment of HIV infections, this oral dual CCR2 and CCR5 antagonist reversed liver fibrosis in patients with MASH [155]. However, novel data relativize this finding in MASH [156]. |
ACC: acetyl-CoA carboxylase; CCR: chemokine CC motif receptor; FGF: fibroblast growth factor; FXR: farnesoid X-activated receptor; PPAR: peroxisome proliferator-activated receptor; SCD1: stearoyl-CoA desaturase 1; THRβ: thyroid hormone receptor-β
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1001334_sup_1.pdf.
The graphical abstract was generated using Servier Medical Art, provided by Servier. The authors would like to express their gratitude to Sabine Weiskirchen from the University Hospital Aachen for preparing Figure 2 of this review. Additionally, we would like to extend our sincere thanks to the World Obesity Federation for assisting and granting permission to publish Figure 1B and the Supplementary material.
AI and AI-assisted Technologies: The authors of this article used the Large Language Model RWTHgpt by RWTH Aachen University exclusively for minor translations and grammatical corrections in this work. All sentences revised by RWTHgpt were reviewed and verified by the authors.
AL and RW: Conceptualization, Writing—original draft, Writing—review & editing. Both authors read and approved the submitted version.
Amedeo Lonardo who is the Associate Editor of Exploration of Medicine had no involvement in the decision-making or the review process of this manuscript. Another author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.