Affiliation:
1Department of Internal Medicine, Azienda Ospedaliero-Universitaria di Modena (–2023), Modena, 41100, Italy
†These authors share the first authorship.
Email: a.lonardo@libero.it
ORCID: https://orcid.org/0000-0001-9886-0698
Affiliation:
2MAFLD Research Center, Department of Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang, China
3Key Laboratory of Diagnosis and Treatment for the Development of Chronic Liver Disease in Zhejiang Province, Wenzhou 325000, Zhejiang, China
†These authors share the first authorship.
ORCID: https://orcid.org/0000-0003-4984-2631
Explor Dig Dis. 2025;4:100577 DOl: https://doi.org/10.37349/edd.2025.100577
Received: May 07, 2025 Accepted: June 12, 2025 Published: June 18, 2025
Academic Editor: Oren Tirosh, Hebrew University of Jerusalem, Israel
Yu JW et al. (World J Gastroenterol. 2025;31:105188. DOI: 10.3748/wjg.v31.i16.105188) used male Sprague-Dawley rats fed a high-fat diet for 8 weeks to recapitulate metabolic dysfunction-associated steatotic liver disease (MASLD) experimentally. MASLD rats were randomized to receive either the duodenal mucosal ablation (DMA) using irreversible electroporation (IRE) during laparotomy or sham DMA. Data have shown that DMA was associated with duodenal thickening compared to the control group, crypts were narrower and shallower crypts and villi slimmer than sham DMA group. Moreover, the DMA group exhibited improved liver histology compared to the sham group though accompanied by inconsistent variations in blood lipid values and statistically non-significant variations in surrogate indices of MASLD. Thirdly, DMA rats had lower serum concentrations of gut hormones with crucial metabolic functions, lower lipopolysaccharide serum level, increased duodenal expression and immunofluorescence staining intensity of gut hormones expression, and higher expression of zonula occludens-1 and claudin than sham-rats. The study by Yu, et al. has innovative findings and is properly designed to illustrate the pathomechanisms underlying improved MASLD histology after DMA with IRE. However, this paper also has some methodological limitations that prompt additional studies in animal models and, ideally, in humans to be conducted as soon as safety and feasibility are demonstrated.
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease, defines steatotic liver disease (SLD) in the presence of at least one cardiometabolic risk factor without any harmful alcohol intake [1]. The MASLD spectrum comprises simple steatosis, metabolic dysfunction-associated steatohepatitis [(MASH), previously known as nonalcoholic steatohepatitis (NASH)], fibrosis, cirrhosis with or without hepatocellular carcinoma [1, 2]. Collectively, MASLD poses a heavy clinical and epidemiological burden against which limited therapeutic strategies are available [2–4]. The pathogenesis of MASLD is complex and multi-factorial, with various noxious stimuli interacting in parallel to contribute to liver injury in the context of systemic metabolic dysfunction [5]. Among such noxious stimuli, type 2 diabetes (T2D) plays a major role given that T2D facilitates development and progression of MASLD and that MASLD, in turn, worsens glucose homeostasis [6]. Therefore, MASLD is a systemic condition and requires a holistic approach [4, 7]. Sex differences and liver fibrosis are major determinants of the whole spectrum of hepatic and extrahepatic manifestations of MASLD [4, 8, 9], accounting for the importance in this field of sex-specific analysis of data and of animal models faithfully recapitulating fibrosing disease such as seen in human disease.
Various organs comprising not only the liver but several extrahepatic organs such as the pancreas and the adipose tissue, kidneys, the skeletal muscle, and duodenum regulate metabolic physiology and participate in the development of systemic metabolic dysregulation. Among these, the duodenum is not universally appreciated as a key metabolic organ. Instead, several lines of evidence fully support its role in the physiopathology of metabolism. Evidence has shown that prolonged fasting will induce atrophy of duodenal mucosa with severe ultrastructural changes in a proportion of cases [10] and that, conversely, obesogenic diets rich in simple sugars and lipids will promote hyperplastic changes of proximal small bowel mucosa in rodents associated with fewer enteroendocrine cells [11].
Figure 1 summarizes the role of duodenum in the regulation of insulin sensitivity and development of metabolic dysfunction [12].
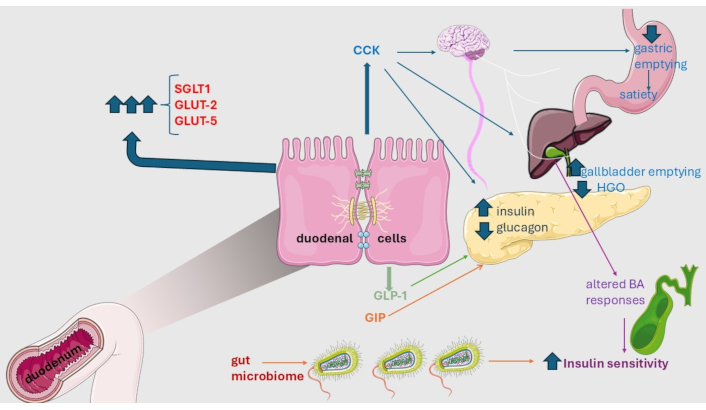
Metabolic role of duodenum. Duodenal mucosa regulates insulin sensitivity through its secreted hormones cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1) that govern satiety as well as the release of pancreatic hormones directly or per vagal route. Similarly, the glucose-dependent insulinotropic peptide (GIP) increases the secretion of insulin and glucagon and decreases the hepatic glucose output (HGO). Additional mechanisms potentially involved in down-regulation of insulin resistance comprise altered bile acid (BA) response in the post-prandial phase and variations in the composition of the intestinal microbiota. Finally, metabolic dysfunction in type 2 diabetes and other conditions is associated with a marked increase in the intestinal transporters sodium-glucose co-transporter-1 (SGLT1), glucose transporter 2 (GLUT-2), and glucose transporter 5 (GLUT-5) which will potentially result in increased nutrient absorption and ongoing metabolic dysfunction. Original illustration provided by or modified from Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
Collectively, these findings provide the rationale for manipulating the duodenal mucosa to modify the absorption of nutrients in the context of metabolic disorders [12]. Various surgical and endoscopic approaches may be used to this end [13–15].
With this intriguing backset, next, we summarize and comment on a recent study by Yu et al. [16] by pinpointing novel findings, and highlighting points of strength and methodological limitations of this investigation.
Yu et al. [16] utilized male Sprague-Dawley rats fed a high-fat diet for 8 weeks to recapitulate MASLD experimentally. MASLD rats were randomly categorized into the Duodenal mucosal ablation (DMA group, n = 6) and the sham-control group (non-DMA group, n = 6). DMA was carried out using irreversible electroporation (IRE) obtained with a three-electrode ablation catheter (diameter: 2.5 mm) ending with a temperature sensor, and a square-wave pulse generator to generate a burst of electric pulses. The ablation catheter was positioned intraduodenally through pylorus. After the duodenum was ablated, the electrode catheter was withdrawn, and the abdominal incision was sutured. Postoperatively, rats were injected with buprenorphine and cefuroxime intraperitoneally and sacrificed after 2 weeks.
Three principal lines of results were documented after DMA.
Firstly, as regards the duodenum, 2 weeks after DMA, the duodenal mucosa was macroscopically healed both macroscopically and histologically, without any inflammatory, ulcerative, or stenosing complications. Moreover, although DMA was associated with significant thickening of each duodenal layer compared to the control group (P < 0.05), crypts were significantly narrower and shallower crypts and villi significantly slimmer (P < 0.05 for all comparisons) vs. non-DMA group.
Secondly, while the sham group exhibited SLD, with lipid droplets of different sizes and disorganized hepatocyte structure, liver histology was significantly improved in the DMA group. However, these histological improvements were accompanied by inconsistent variations in blood lipid values (serum total cholesterol and low-density-lipoprotein cholesterol being significantly lower whereas serum triglycerides, free fatty acids, and high-density-lipoprotein cholesterol were higher in the DMA than in the sham-control group) and statistically non-significant variations in surrogate indices of MASLD such as transaminases and total bile acids.
Thirdly, the DMA group exhibited significantly lower serum concentrations of gut hormones [glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and cholecystokinin (CCK)] with crucial metabolic functions (e.g., intake and absorption of nutrients, regulation of glucose homeostasis, secretion of pancreatic juice, and gastric and gallbladder emptying) than those in the control group (P < 0.05). Moreover, the DMA animals had significantly lower lipopolysaccharide serum levels and significantly increased duodenal expression and immunofluorescence staining intensity of GLP-1, GIP, and CCK, and higher expression of zonula occludens-1, and claudin than in the sham-control group. Collectively, these findings suggest decreased intestinal permeability after DMA.
In humans, individuals with T2D, the prototypic metabolic disorder, which is often associated with obesity, have jejunal mucosa hypertrophy, hyperplasia of enteroendocrine cells, and increased numbers of enteroendocrine cells and enterocytes compared to nondiabetic individuals; moreover, variations in glucose transporters, enteric nerves, and intestinal microbiota composition have also been reported [12]. Clinically, the best-studied endoscopic procedure is duodenal mucosa resurfacing (DMR) which is based on the principle that resurfacing the mucosal interface after endoscopic resection will reset and correct any abnormal signaling from the duodenal mucosa and will, therefore, result in improved pancreatic endocrine function and glucose tolerance owing to restored normal mucosa surface [12]. The endoscopic procedure of DMR is safe and well tolerated. However, additional studies are needed to demonstrate its validity irrespective of concurrently administered antidiabetic medications to obtain the reversal of T2D and improvement in liver histology [12]. MASLD is tightly and bi-directionally associated with T2D and it is therefore logical to speculate that DMR may also be useful in MASLD treatment irrespective of how it is implemented, namely with thermal ablation or with IRE.
The study by Yu et al. [16] has innovative findings regarding the safety and effectiveness of DMA with IRE to contrast MASLD in an experimental rat model. This is a conceptually intriguing and potentially clinically relevant research avenue. Moreover, the study by Yu et al. [16] is properly designed to illustrate the changes in gut hormones and intestinal permeability underlying this improved liver histology. However, this paper also has some methodological limitations that prompt additional studies.
One fundamental research question is whether (and to what extent) murine MASLD models faithfully recapitulate human disease. It is now clear that these models rarely show MASH with significant fibrosis (≥ F2), which typically requires protracted observations, diets including high cholesterol content, and, often, also the use of genetically engineered animals [17]. Furthermore, in the study by Yu et al. [16] only male animals have been used, which conflicts with recommendations to characterize sex differences in MASLD pathobiology and treatment [18].
Additionally, no information is provided regarding the temperature of the housing. This is important given that thermoneutral housing (i.e., a temperature where mice do not need to expend energy to maintain their core body temperature) exacerbates MASLD in mouse models, leading to more severe liver histology and even enabling female mice to develop obesity and MASLD [19].
It is also pinpointed that the procedure of DMA with IRE reported by Yu et al. [16] is extremely invasive which raises ethical concerns pertaining to the causation of unnecessary suffering to animals. Moreover, the surgical procedure involves the utilization of drugs (e.g., antibiotics and analgesics) which may potentially alter the physiology of the gastrointestinal tract by modifying the eating habits of rats owing to satiety, anorexia, vomiting, and potentially altering intestinal microbiota diversity in these animals.
All these limitations may be overcome by using animal models in which DMA with IRE may be performed endoscopically [20]. However, considering the many limitations in MASLD animal models, it will be important to study endoscopic IRE in humans as soon as sufficient evidence of safety and feasibility is obtained in preliminary studies.
CCK: cholecystokinin
DMA: duodenal mucosal ablation
DMR: duodenal mucosa resurfacing
GIP: glucose-dependent insulinotropic polypeptide
GLP-1: glucagon-like peptide-1
IRE: irreversible electroporation
MASH: metabolic dysfunction-associated steatohepatitis
MASLD: metabolic dysfunction-associated steatotic liver disease
SLD: steatotic liver disease
T2D: type 2 diabetes
AL and MHZ: Conceptualization, Visualization, Writing—original draft, Writing—review & editing.
Prof. Amedeo Lonardo serves as an Associate Editor and Guest Editor of Exploration of Digestive Diseases. However, he was not involved in any way in the peer review process nor in decision-making of this manuscript, which were instead independently conducted by the journal. MHZ has received honoraria for lectures from AstraZeneca, Hisky Medical Technologies, and Novo Nordisk, consulting fees from Boehringer Ingelheim.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1484
Download: 21
Times Cited: 0
