Exploring evidence-based innovative therapy for the treatment of chronic HBV infection: experimental and clinical
With the advent of various vaccines and antimicrobial agents during the 20th century, the control and containment of infectious diseases appeared to be a matter of time. However, studies unveiled the diverse natures of microbes, t
[...] Read more.
With the advent of various vaccines and antimicrobial agents during the 20th century, the control and containment of infectious diseases appeared to be a matter of time. However, studies unveiled the diverse natures of microbes, their lifestyle, and pathogenetic potentials. Since the ground-breaking discovery of the hepatitis B virus (HBV) by Baruch Blumberg and the subsequent development of a vaccine in the early 1980s, the main task of the scientific community has been to develop a proper management strategy for HBV-induced chronic liver diseases. In the early 1980’s, standard interferon (IFN) induced a reduction of HBV DNA levels, followed by the normalization of serum transaminases (alanine aminotransferase, ALT), in some chronic hepatitis B (CHB) patients. However, in the course of time, the limitations of standard IFN became evident, and the search for an alternative began. In the late 1980’s, nucleoside analogs entered the arena of CHB treatment as oral drugs with potent antiviral capacities. At the beginning of the 21st century, insights were developed into the scope and limitations of standard IFN, pegylated-IFN as well as nucleoside analogs for treating CHB. Considering the non-cytopathic nature of the HBV, the presence of covalently closed circular DNA (cccDNA) in the nucleus of the infected hepatocytes and HBV-induced immune-mediated liver damages, a new field of CHB management was initiated by modulating the hosts’ immune system through immune therapy. This review will discuss the nature and design of innovative immune therapy for CHB.
Sheikh Mohammad Fazle Akbar ... Yoichi Hiasa
View:1896
Download:29
Times Cited: 0
With the advent of various vaccines and antimicrobial agents during the 20th century, the control and containment of infectious diseases appeared to be a matter of time. However, studies unveiled the diverse natures of microbes, their lifestyle, and pathogenetic potentials. Since the ground-breaking discovery of the hepatitis B virus (HBV) by Baruch Blumberg and the subsequent development of a vaccine in the early 1980s, the main task of the scientific community has been to develop a proper management strategy for HBV-induced chronic liver diseases. In the early 1980’s, standard interferon (IFN) induced a reduction of HBV DNA levels, followed by the normalization of serum transaminases (alanine aminotransferase, ALT), in some chronic hepatitis B (CHB) patients. However, in the course of time, the limitations of standard IFN became evident, and the search for an alternative began. In the late 1980’s, nucleoside analogs entered the arena of CHB treatment as oral drugs with potent antiviral capacities. At the beginning of the 21st century, insights were developed into the scope and limitations of standard IFN, pegylated-IFN as well as nucleoside analogs for treating CHB. Considering the non-cytopathic nature of the HBV, the presence of covalently closed circular DNA (cccDNA) in the nucleus of the infected hepatocytes and HBV-induced immune-mediated liver damages, a new field of CHB management was initiated by modulating the hosts’ immune system through immune therapy. This review will discuss the nature and design of innovative immune therapy for CHB.
 Reactive oxygen species may influence on the crossroads of stemness, senescence, and carcinogenesis in a cell via the roles of APRO family proteinsOpen AccessPerspectiveExcessive reactive oxygen species (ROS) may cause oxidative stress which is involved in aging and in the pathogenesis of various human diseases. Whereas unregulated levels of the ROS may be harmful, regulated basal level of ROS is [...] Read more.Yuka Ikeda ... Satoru MatsudaPublished: October 31, 2021 Explor Med. 2021;2:443–454
Reactive oxygen species may influence on the crossroads of stemness, senescence, and carcinogenesis in a cell via the roles of APRO family proteinsOpen AccessPerspectiveExcessive reactive oxygen species (ROS) may cause oxidative stress which is involved in aging and in the pathogenesis of various human diseases. Whereas unregulated levels of the ROS may be harmful, regulated basal level of ROS is [...] Read more.Yuka Ikeda ... Satoru MatsudaPublished: October 31, 2021 Explor Med. 2021;2:443–454 Separating the apples from the oranges: from NAFLD heterogeneity to personalized medicineOpen AccessCommentaryRecently, Arrese and Colleagues have published a review article entitled, “Insights into Nonalcoholic Fatty-Liver Disease (NAFLD) Heterogeneity” (Semin Liver Dis. 2021;41:421–34. doi: 10.1055/s-0041-1730927). This milestone [...] Read more.Amedeo LonardoPublished: October 31, 2021 Explor Med. 2021;2:435–442
Separating the apples from the oranges: from NAFLD heterogeneity to personalized medicineOpen AccessCommentaryRecently, Arrese and Colleagues have published a review article entitled, “Insights into Nonalcoholic Fatty-Liver Disease (NAFLD) Heterogeneity” (Semin Liver Dis. 2021;41:421–34. doi: 10.1055/s-0041-1730927). This milestone [...] Read more.Amedeo LonardoPublished: October 31, 2021 Explor Med. 2021;2:435–442 Aortic complications in pregnancy: the less remembered chapter—a narrative reviewOpen AccessReviewPregnancy increases the risk of common vascular events and also the rarer events like aortic dissection (AD)/aortic rupture and this is even more pronounced in patients with predisposing aortopathies. AD was found to occur in 0.00 [...] Read more.Preetha Rajasekaran ... Bashi V. VelayudhanPublished: October 31, 2021 Explor Med. 2021;2:423–434
Aortic complications in pregnancy: the less remembered chapter—a narrative reviewOpen AccessReviewPregnancy increases the risk of common vascular events and also the rarer events like aortic dissection (AD)/aortic rupture and this is even more pronounced in patients with predisposing aortopathies. AD was found to occur in 0.00 [...] Read more.Preetha Rajasekaran ... Bashi V. VelayudhanPublished: October 31, 2021 Explor Med. 2021;2:423–434 A review of aortic thrombosis in COVID-19 infectionOpen AccessMeta-AnalysisAim: As the novel coronavirus disease 2019 (COVID-19) pandemic impacts the global healthcare system, evolving data show increased frequency of arterial and venous thromboembolism among patients with COVID-19 infection. Aortic t [...] Read more.Korin Karabulut ... James MorganPublished: October 31, 2021 Explor Med. 2021;2:410–422
A review of aortic thrombosis in COVID-19 infectionOpen AccessMeta-AnalysisAim: As the novel coronavirus disease 2019 (COVID-19) pandemic impacts the global healthcare system, evolving data show increased frequency of arterial and venous thromboembolism among patients with COVID-19 infection. Aortic t [...] Read more.Korin Karabulut ... James MorganPublished: October 31, 2021 Explor Med. 2021;2:410–422 Exploring evidence-based innovative therapy for the treatment of chronic HBV infection: experimental and clinicalOpen AccessReviewWith the advent of various vaccines and antimicrobial agents during the 20th century, the control and containment of infectious diseases appeared to be a matter of time. However, studies unveiled the diverse natures of microbes, t [...] Read more.Sheikh Mohammad Fazle Akbar ... Yoichi HiasaPublished: October 31, 2021 Explor Med. 2021;2:392–409
Exploring evidence-based innovative therapy for the treatment of chronic HBV infection: experimental and clinicalOpen AccessReviewWith the advent of various vaccines and antimicrobial agents during the 20th century, the control and containment of infectious diseases appeared to be a matter of time. However, studies unveiled the diverse natures of microbes, t [...] Read more.Sheikh Mohammad Fazle Akbar ... Yoichi HiasaPublished: October 31, 2021 Explor Med. 2021;2:392–409 Objective measurement of sleep by smartphone application: comparison with actigraphy and relation to self-reported sleepOpen AccessOriginal ArticleAim: Smartphone technology is increasingly used by the public to assess sleep. Specific features of some sleep-tracking applications are comparable to actigraphy in objectively monitoring sleep. The clinical utility of smartpho [...] Read more.Taylor Maynard ... Sandy NeargarderPublished: October 31, 2021 Explor Med. 2021;2:382–391
Objective measurement of sleep by smartphone application: comparison with actigraphy and relation to self-reported sleepOpen AccessOriginal ArticleAim: Smartphone technology is increasingly used by the public to assess sleep. Specific features of some sleep-tracking applications are comparable to actigraphy in objectively monitoring sleep. The clinical utility of smartpho [...] Read more.Taylor Maynard ... Sandy NeargarderPublished: October 31, 2021 Explor Med. 2021;2:382–391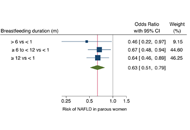 Breastfeeding duration and reduced risk of NAFLD in midlife of parous womenOpen AccessCommentaryAlessandro Mantovani ... Andrea DalbeniPublished: October 31, 2021 Explor Med. 2021;2:378–381
Breastfeeding duration and reduced risk of NAFLD in midlife of parous womenOpen AccessCommentaryAlessandro Mantovani ... Andrea DalbeniPublished: October 31, 2021 Explor Med. 2021;2:378–381