Let’s review the gut microbiota in systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic, immune-mediated disease associated with significant morbidity and mortality. New evidence suggests that diet, gut microbiota, intestinal permeability
[...] Read more.
Systemic lupus erythematosus (SLE) is a chronic, immune-mediated disease associated with significant morbidity and mortality. New evidence suggests that diet, gut microbiota, intestinal permeability, and endotoxemia may modulate chronic inflammation and disease activity in SLE. This review focus on what is known about the gut microbiota in lupus mouse models and SLE patients and the possible mechanisms that connect the gut microbiota with SLE. It included 29 studies (12 animal studies, 15 human studies, and 2 included data on both), with variable results regarding alpha and beta-diversity and gut microbiota composition between lupus-mouse models and SLE patients. Ruminococcus (R.) gnavus was significantly increased in lupus nephritis (LN) in one study, but this was not corroborated by others. Despite the different results, mechanistic lupus mouse model studies have shown that gut microbiota can modulate disease activity. Interestingly, pathobiont translocation in monocolonized and autoimmune-prone mice induced autoantibodies and caused mortality, which could be prevented by a vaccine targeting the pathobiont. Moreover, studies on fecal transplants and diet on different lupus mouse models showed an effect on disease activity. In SLE patients, a higher adherence to the Mediterranean diet was associated with lower disease activity, which may be explained by the connection between diet and gut microbiota. Although gut dysbiosis has been observed in SLE patients and lupus mouse models, it remains to clarify if it is a cause or a consequence of the disease or its treatments. Further studies with larger and well-characterized populations will undoubtedly contribute to deciphering the role of gut microbiota in SLE development, progression, and outcome.
Inês Almada-Correia ... João Eurico Fonseca
View:1146
Download:24
Times Cited: 0
Systemic lupus erythematosus (SLE) is a chronic, immune-mediated disease associated with significant morbidity and mortality. New evidence suggests that diet, gut microbiota, intestinal permeability, and endotoxemia may modulate chronic inflammation and disease activity in SLE. This review focus on what is known about the gut microbiota in lupus mouse models and SLE patients and the possible mechanisms that connect the gut microbiota with SLE. It included 29 studies (12 animal studies, 15 human studies, and 2 included data on both), with variable results regarding alpha and beta-diversity and gut microbiota composition between lupus-mouse models and SLE patients. Ruminococcus (R.) gnavus was significantly increased in lupus nephritis (LN) in one study, but this was not corroborated by others. Despite the different results, mechanistic lupus mouse model studies have shown that gut microbiota can modulate disease activity. Interestingly, pathobiont translocation in monocolonized and autoimmune-prone mice induced autoantibodies and caused mortality, which could be prevented by a vaccine targeting the pathobiont. Moreover, studies on fecal transplants and diet on different lupus mouse models showed an effect on disease activity. In SLE patients, a higher adherence to the Mediterranean diet was associated with lower disease activity, which may be explained by the connection between diet and gut microbiota. Although gut dysbiosis has been observed in SLE patients and lupus mouse models, it remains to clarify if it is a cause or a consequence of the disease or its treatments. Further studies with larger and well-characterized populations will undoubtedly contribute to deciphering the role of gut microbiota in SLE development, progression, and outcome.
 Quality by design based development of nanostructured lipid carrier: a risk based approachOpen AccessReviewThe aim of this review is to discuss the development of nanostructured lipid carrier (NLC) by the application of quality by design (QbD). QbD started with the evolution of the quality concept and sl [...] Read more.Tausif AlamPublished: December 31, 2022 Explor Med. 2022;3:617–638
Quality by design based development of nanostructured lipid carrier: a risk based approachOpen AccessReviewThe aim of this review is to discuss the development of nanostructured lipid carrier (NLC) by the application of quality by design (QbD). QbD started with the evolution of the quality concept and sl [...] Read more.Tausif AlamPublished: December 31, 2022 Explor Med. 2022;3:617–638 Antioxidant and photoprotective potential of Polypodium leucotomosOpen AccessReviewIn recent years, Polypodium leucotomos has emerged with a great interest for having medicinal and therapeutic potential. It is producing very promising results due to the presence of antioxidant and [...] Read more.Rosy Yesela Mancilla Santa Cruz ... María Segunda Aurora PradoPublished: December 29, 2022 Explor Med. 2022;3:607–616
Antioxidant and photoprotective potential of Polypodium leucotomosOpen AccessReviewIn recent years, Polypodium leucotomos has emerged with a great interest for having medicinal and therapeutic potential. It is producing very promising results due to the presence of antioxidant and [...] Read more.Rosy Yesela Mancilla Santa Cruz ... María Segunda Aurora PradoPublished: December 29, 2022 Explor Med. 2022;3:607–616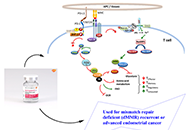 The journey of dostarlimab: a successful weapon for cancer treatmentOpen AccessReviewIn a number of malignancies, new immuno-oncology therapies that focus on the programmed cell death 1 (PD-1) have improvised the patient condition along with a positive aftereffect. Monoclonal antibo [...] Read more.Rouchan Ali ... Pooja A. ChawlaPublished: December 29, 2022 Explor Med. 2022;3:592–606
The journey of dostarlimab: a successful weapon for cancer treatmentOpen AccessReviewIn a number of malignancies, new immuno-oncology therapies that focus on the programmed cell death 1 (PD-1) have improvised the patient condition along with a positive aftereffect. Monoclonal antibo [...] Read more.Rouchan Ali ... Pooja A. ChawlaPublished: December 29, 2022 Explor Med. 2022;3:592–606 Right atrial thrombus, junctional tachycardia, and critical lower limb ischemia: three rare complications of severe acute respiratory syndrome coronavirus 2 infectionOpen AccessCase ReportIn recent years, Polypodium leucotomos has emerged with a great interest for having medicinal and therapeutic potential. It is producing very promising results due to the presence of antioxidant and [...] Read more.Said Makani ... Youssef TijaniPublished: December 29, 2022 Explor Med. 2022;3:583–591
Right atrial thrombus, junctional tachycardia, and critical lower limb ischemia: three rare complications of severe acute respiratory syndrome coronavirus 2 infectionOpen AccessCase ReportIn recent years, Polypodium leucotomos has emerged with a great interest for having medicinal and therapeutic potential. It is producing very promising results due to the presence of antioxidant and [...] Read more.Said Makani ... Youssef TijaniPublished: December 29, 2022 Explor Med. 2022;3:583–591 Predictive value of SII and sd-LDL for contrast-induced acute kidney injury in STEMI patients undergoing percutaneous coronary interventionOpen AccessOriginal ArticleAim: To investigate the relationship between the incidence of contrast-induced acute kidney injury (CI-AKI) and the level of small dense low-density lipoprotein (sd-LDL) and systemic immune-infla [...] Read more.Guoqi Shen ... Wenhua LiPublished: December 28, 2022 Explor Med. 2022;3:571–582
Predictive value of SII and sd-LDL for contrast-induced acute kidney injury in STEMI patients undergoing percutaneous coronary interventionOpen AccessOriginal ArticleAim: To investigate the relationship between the incidence of contrast-induced acute kidney injury (CI-AKI) and the level of small dense low-density lipoprotein (sd-LDL) and systemic immune-infla [...] Read more.Guoqi Shen ... Wenhua LiPublished: December 28, 2022 Explor Med. 2022;3:571–582 An unusual presentation of Whipple’s disease: adenopathies, polyarthralgia and dermatomyositis-like symptomsOpen AccessCase ReportWhipple’s disease (WD) is a rare systemic disease caused by gram-positive bacillus bacteria that invades multiple organs mainly the intestinal epithelium. Its manifestation is not only l [...] Read more.Randa Choueiry ... Joseph AmaraPublished: December 27, 2022 Explor Med. 2022;3:561–570
An unusual presentation of Whipple’s disease: adenopathies, polyarthralgia and dermatomyositis-like symptomsOpen AccessCase ReportWhipple’s disease (WD) is a rare systemic disease caused by gram-positive bacillus bacteria that invades multiple organs mainly the intestinal epithelium. Its manifestation is not only l [...] Read more.Randa Choueiry ... Joseph AmaraPublished: December 27, 2022 Explor Med. 2022;3:561–570 Let’s review the gut microbiota in systemic lupus erythematosusOpen AccessReviewSystemic lupus erythematosus (SLE) is a chronic, immune-mediated disease associated with significant morbidity and mortality. New evidence suggests that diet, gut microbiota, intestinal permeability [...] Read more.Inês Almada-Correia ... João Eurico FonsecaPublished: December 26, 2022 Explor Med. 2022;3:540–560
Let’s review the gut microbiota in systemic lupus erythematosusOpen AccessReviewSystemic lupus erythematosus (SLE) is a chronic, immune-mediated disease associated with significant morbidity and mortality. New evidence suggests that diet, gut microbiota, intestinal permeability [...] Read more.Inês Almada-Correia ... João Eurico FonsecaPublished: December 26, 2022 Explor Med. 2022;3:540–560 Treatment opportunities with Fernandoa adenophylla and recent novel approaches for natural medicinal phytochemicals as a drug delivery systemOpen AccessReviewFernandoa adenophylla (FA, Heterophragma adenophyllum) is a plant, cultivated throughout Africa and Southeast Asia. It contains potent phytochemicals such as novel naphthoquinones, their derivatives [...] Read more.Sangeet Kumar Mall ... Md Sabir AlamPublished: November 14, 2022 Explor Med. 2022;3:516–539
Treatment opportunities with Fernandoa adenophylla and recent novel approaches for natural medicinal phytochemicals as a drug delivery systemOpen AccessReviewFernandoa adenophylla (FA, Heterophragma adenophyllum) is a plant, cultivated throughout Africa and Southeast Asia. It contains potent phytochemicals such as novel naphthoquinones, their derivatives [...] Read more.Sangeet Kumar Mall ... Md Sabir AlamPublished: November 14, 2022 Explor Med. 2022;3:516–539