Affiliation:
1Lviv Oncologic Regional Treatment and Diagnostic Center, 79031 Lviv, Ukraine
2Ivano-Frankivsk National Medical University, 76000 Ivano-Frankivsk, Ukraine
Email: nkitsera@gmail.com
ORCID: https://orcid.org/0000-0002-6617-9336
Affiliation:
3Lviv Polytechnic National University, 79000 Lviv, Ukraine
ORCID: https://orcid.org/0000-0002-2157-1422
Affiliation:
1Lviv Oncologic Regional Treatment and Diagnostic Center, 79031 Lviv, Ukraine
ORCID: https://orcid.org/0000-0003-2074-6320
Affiliation:
1Lviv Oncologic Regional Treatment and Diagnostic Center, 79031 Lviv, Ukraine
ORCID: https://orcid.org/0000-0003-0401-8796
Explor Med. 2025;6:1001343 DOl: https://doi.org/10.37349/emed.2025.1001343
Received: February 10, 2025 Accepted: June 25, 2025 Published: July 09, 2025
Academic Editor: Haim Werner, Tel Aviv University, Israel
Aim: To analyze cancer cases among male long-lived in the Lviv region (Ukraine) during 1991–2019.
Methods: Our retrospective study analyzed cancer cases among 312 males aged 90+ from the Lviv region (Ukraine) during 1991–2019 using data from the Cancer Registry of Lviv Oncologic Regional Treatment and Diagnostic Center.
Results: Among 312 long-lived men, 25 types of cancer were diagnosed (median age 92.5 ± 2 years): skin cancer (30.77%), prostate cancer (19.87%), and stomach cancer (7.69%) were the most common. A higher proportion of cancer patients lived in urban areas (72.12%) compared to rural areas (27.88%), indicating better healthcare access in urban areas, which may contribute to better survival rates. A strong negative correlation was found between age and survival duration, with a Pearson’s correlation coefficient (r) of −0.93. As age increases, the number of long-lived and the number of cancer diagnoses decreases, especially after age 93. Early-stage cancers (Stages 0 and I) showed significantly better survival outcomes, with 100% survival for 3–4 years in Stage 0, while Stage IV had a 78.13% mortality rate within one year, with no survivors beyond three years. For patients over 96, survival beyond one year is rare, indicating the need for a palliative care approach focused on symptom management rather than curative treatment.
Conclusions: Future research should focus on improving early cancer detection for elderly patients, developing tailored treatments for aging individuals, and improving healthcare access for rural populations.
The study of cancer prevalence among male long-lived in the Lviv region for the period 1991–2019 is highly relevant, considering population aging trends and increasing cancer incidence rates. According to data, individuals aged 65 and older bear the primary burden of cancer in developed countries [1, 2]. Ukraine, and specifically the Lviv region, demonstrates a similar demographic situation, highlighting the need to study cancer risks among elderly men.
Aging is a key risk factor for cancer development [3]. With age, somatic mutations accumulate, increasing the likelihood of malignant neoplasms. Furthermore, aging is accompanied by tissue and immune system changes that may contribute to cancer development [4, 5].
European studies indicate that increasing life expectancy leads to a rise in cancer cases among older individuals [6]. Specifically, over 4 million new cancer cases were recorded in Europe in 2020, a significant portion of which occurred in older adults. This underscores the importance of developing effective strategies for cancer prevention and treatment in the context of population aging [7].
In the Lviv region, as in other regions of Ukraine, the proportion of elderly individuals in the overall population structure is increasing. This necessitates a detailed analysis of the prevalence and course of cancer among male long-lived, which could inform the development of targeted medical and social programs.
Aim of our study is to analyze cancer cases among male long-lived in the Lviv region (Ukraine) during 1991–2019, taking into account place of residence, type of treatment, cancer stage, and survival duration after diagnosis.
Following ethical approval (No. 03/25-3, dated March 19, 2025, from the Lviv Oncologic Regional Treatment and Diagnostic Center, Ukraine), we conducted a retrospective study analyzing cancer cases among male long-lived aged over 90 in urban and rural areas of the Lviv region (Western Ukraine). The study covered the period from January 1, 1991, to December 31, 2019, and included individuals born between 1901 and 1929. Data on 312 cases of malignant neoplasms in long-lived men were collected and organized into a database maintained by the Cancer Registry of the Lviv Regional Oncology Medical Diagnostic Centre which included demographic details, cancer stages, treatment methods and survival outcomes. Individual patient consent was not required, as the study was based on retrospective analysis of anonymized data collected for public health and epidemiological purposes. The registry contains no personally identifiable information, and all data were fully de-identified prior to researcher access. The institutional ethics committee confirmed that the use of such anonymized data does not pose any risk to individual privacy and complies with national and international ethical standards for research involving human subjects. Therefore, the requirement for informed consent from each patient was waived.
According to demographic data, the population of the Lviv region in 2019 was 2,522,000, with 61% living in urban areas and 39% in villages. The cohort consisted of 312 patients, with 72.12% living in towns and 27.88% residing in villages. This disparity partly reflects the real demographic situation in Lviv region, but may also indicate limited access to health services in rural areas, leading to fewer diagnosed cancer cases among the rural population. Age distribution was as follows: 64.74% aged 90–92, 27.88% aged 93–95, 6.41% aged 96–98, and 0.96% aged 99–100. Cancer stages were categorized as Stage 0 to Stage IV or not set, with most cases falling into Stages I (24.04%) and II (30.77%). The compiled data and analyses offer insights into cancer prevalence and survival trends among elderly male cancer patients in the Lviv region, emphasizing the need for targeted medical and social interventions.
To analyze the collected data, we employed a combination of descriptive and analytic epidemiology statistical methods. Descriptive statistics were used to summarize the frequency distribution of cancer cases by age group, cancer stage, and survival duration categories. Percentages were calculated to highlight trends in survival outcomes across different demographics. Pearson’s correlation coefficient (r) was applied to assess the relationship between age and survival duration, enabling us to determine statistical associations within the cohort [8]. Statistical analysis was performed using Microsoft Excel 2013.
A combinatorial grouping method was applied to analyze relationships between demographic and clinical variables, with age groups (90–91, 92–93, 94–95, 96–97, over 98 years) serving as the constant variable across all combinations. This approach examined links between age and survival duration, survival based on cancer stage at diagnosis, and the distribution of patients by residence and age. It provided a structured framework to identify multidimensional patterns and better understand survival trends among long-lived cancer patients.
Among 312 long-lived men were diagnosed 25 types of cancer. Table 1 shows the distribution of long-lived men by age and the most commonly observed ICD-10 codes for malignant neoplasms in Lviv region, Ukraine, spanning the years 1991 to 2019. The most commonly diagnosed cancer is skin cancer (ICD-10 code C44), which accounts for 30.77% of cases overall, with a significant drop in the later years. Prostate cancer (C61) follows, representing 19.87% of the total cases, with the highest frequency in age 90–91 years. Other common cancers include those of the stomach (C16), bladder (C67), and bronchus/lung (C34), though these also show a decrease over age. It reveals a general trend of declining cancer diagnoses over age, particularly in the later periods of the study (Table 1). The data highlights the variation in cancer prevalence across different age groups and the changing patterns of diagnosis in the region.
Distribution of male long-livers by age and the most common types of cancer in Lviv region (Ukraine), 1991–2019
| No | Code of ICD-10 | Diagnosis of malignant neoplasms | Years | All | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 90–91 | 92–93 | 94–95 | 96–97 | > 98 | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||
| 1 | C44 | Other malignant neoplasms of skin | 44 | 14.10 | 29 | 9.29 | 15 | 4.81 | 6 | 1.92 | 2 | 0.64 | 96 | 30.77 |
| 2 | C61 | Prostate | 32 | 10.25 | 19 | 6.09 | 7 | 2.24 | 4 | 1.28 | 0 | 0.00 | 62 | 19.87 |
| 3 | C16 | Stomach | 9 | 2.88 | 5 | 1.60 | 8 | 2.56 | 1 | 0.32 | 1 | 0.32 | 24 | 7.69 |
| 4 | C67 | Bladder | 8 | 2.56 | 9 | 2.88 | 5 | 1.60 | 1 | 0.32 | 0 | 0.00 | 23 | 7.37 |
| 5 | C34 | Bronchus and lung | 5 | 1.60 | 9 | 2.88 | 5 | 1.60 | 1 | 0.32 | 1 | 0.32 | 21 | 6.73 |
| 6 | C18 | Colon | 7 | 2.24 | 5 | 1.60 | 1 | 0.32 | 3 | 0.96 | 0 | 0.00 | 16 | 5.13 |
| 7 | C00 | Lip | 4 | 1.28 | 7 | 2.24 | 2 | 0.64 | 0 | 0.00 | 0 | 0.00 | 13 | 4.17 |
| 8 | C25 | Pancreas | 7 | 2.24 | 5 | 1.60 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 12 | 3.85 |
| 9 | C64 | Kidney | 4 | 1.28 | 2 | 0.64 | 1 | 0.32 | 0 | 0.00 | 1 | 0.32 | 8 | 2.56 |
| 10 | C20 | Rectum | 3 | 0.96 | 2 | 0.64 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 5 | 1.60 |
| 11 | C22 | Liver | 3 | 0.96 | 0 | 0.00 | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 4 | 1.28 |
| 12 | C15 | Oesophagus | 1 | 0.32 | 1 | 0.32 | 0 | 0.00 | 1 | 0.32 | 0 | 0.00 | 3 | 0.96 |
| 13 | C32 | Larynx | 1 | 0.32 | 2 | 0.64 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 3 | 0.96 |
| 14 | C49 | Other connective and soft tissue | 3 | 0.96 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 3 | 0.96 |
| 15 | C60 | Penis | 2 | 0.64 | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 3 | 0.96 |
| 16 | C78 | Secondary malignant neoplasm of respiratory and digestive organs | 1 | 0.32 | 1 | 0.32 | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 3 | 0.96 |
| 17 | C19 | Rectosigmoid junction | 2 | 0.64 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.64 |
| 18 | C50 | Breast | 2 | 0.64 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.64 |
| 19 | C85 | Non-Hodgkin lymphoma | 2 | 0.64 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.64 |
| 20 | C46 | Kaposi’s sarcoma | 1 | 0.32 | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.64 |
| 21 | C48 | Malignant neoplasm of retroperitoneum and peritoneum | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.32 |
| 22 | C74 | Adrenal gland | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.32 |
| 23 | C76 | Malignant neoplasm of other and ill-defined sites | 0 | 0.00 | 0 | 0.00 | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 1 | 0.32 |
| 24 | C83 | Non-follicular lymphoma | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.32 |
| 25 | C91 | Lymphoid leukemia | 0 | 0.00 | 1 | 0.32 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.32 |
| Total | 144 | 46.15 | 99 | 31.73 | 47 | 15.06 | 17 | 5.45 | 5 | 1.60 | 312 | 100 | ||
ICD: International Classification of Diseases
Of the 312 male long-lived with cancer, the majority—225 individuals (72.12%)—lived in urban areas, while 87 individuals (27.88%) resided in rural areas. This may indicate better access to medical services, diagnostics, and treatment in urban settings. The largest age group is 90–91 years, comprising 144 individuals (46.15%), making it the majority among all age groups (Figure 1). Among urban residents, this age group also dominates, with 103 individuals (33.01%). In rural areas, this group accounts for 41 long-lived with cancer (13.14%).
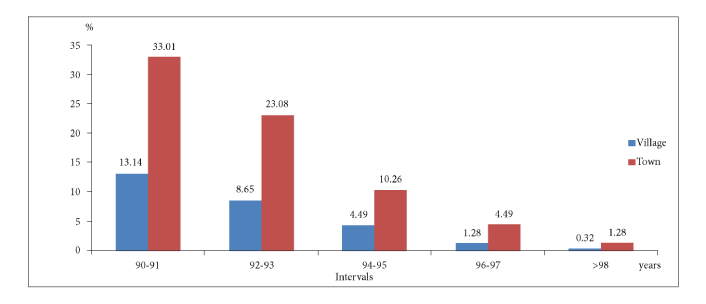
Distribution of long-lived cancer patients by place of residence (urban or rural) and age groups (90–91, 92–93, 94–95, 96-97, > 98 years)
As age increases, the number of cases declines. The 92–93 age group includes 99 individuals (31.73%), dropping to 46 (14.75%) in 94–95 years, 18 (5.77%) in 96–97 years, and 5 (1.60%) in those over 98 years. Among rural residents, these numbers decrease further: 27 (8.65%) in 92–93 years, 14 (4.49%) in 94–95 years, 4 (1.28%) in 96–97 years, and just 1 (0.32%) over 98 years. Urban areas show a similar trend: 72 (23.08%) in 92–93 years, 32 (10.26%) in 94–95 years, 14 (4.49%) in 96–97 years, and 4 (1.28%) over 98 years.
Urban environments likely provide better conditions for cancer diagnosis and treatment, contributing to a higher proportion of long-lived cancer patients. The 90–92 age group remains the largest, emphasizing age as a key factor in longevity. Rural areas have fewer long-lived individuals over 93 years, likely due to limited healthcare access, poorer socio-economic conditions, or harsher living environments. The sharp decline in cases after 93 years, especially in rural areas, suggests greater vulnerability to cancer and lower survival rates post-diagnosis.
The survival durations were grouped into five categories (Figure 2), ranging from less than one year to over six years, and correlated with six cancer stages: Stage 0, Stage I, Stage II, Stage III, Stage IV, and cases where the stage was not determined. The majority of patients (146 cases, 46.79%) succumbed within one year of diagnosis.
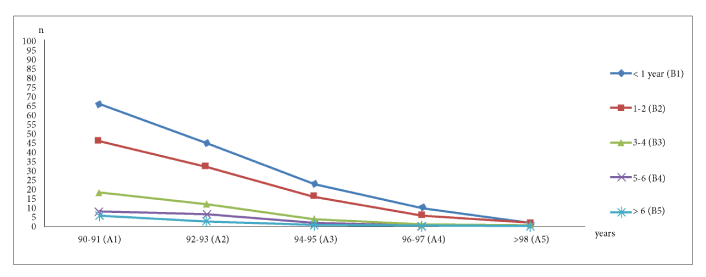
Relationship between age and survival duration in long-lived cancer patients, categorized by age groups (90–91, 92–93, 94–95, 96–97, and over 98 years)
Only 11 patients (3.53%) survived beyond 6 years, highlighting the challenge of long-term survival. Stage 0 cancer was recorded in 1 patient (0.32%) who lived 3–4 years, suggesting early-stage cancers may have favorable outcomes. Stage I included 75 patients (24.04%), with 24 (32%) surviving less than a year, while 6 (8%) lived beyond six years, demonstrating the benefits of early detection.
Stage II was the most common, affecting 96 patients (30.77%); 36 (37.5%) lived less than a year, but 8 (8.33%) survived beyond five years, indicating potential for positive outcomes with intervention. Stage III involved 76 patients (24.36%), with 42 (55.26%) succumbing within a year and none surviving beyond six years, showing the impact of advanced cancer on survival. Stage IV was recorded in 32 patients (10.26%), with 25 (78%) dying within a year and none surviving beyond three years, underscoring its poor prognosis. Of the 32 patients (10.26%) with an undetermined stage, 19 (59.38%) died within a year, with no survivors beyond four years.
Survival duration is strongly linked to cancer stage at diagnosis. Earlier stages (0 and I) show better survival, while advanced stages (III and IV) result in significantly shorter lifespans. In Stage 0, a single patient (0.32%) survived 3–4 years. In Stage I, 24 (32%) succumbed within a year, but 6 (8%) lived beyond six years, reinforcing the importance of early detection. These numbers demonstrate the survival probability:
where:
Pi—percentage of patients surviving within a specific period;
ni—number of patients in the specific period;
Sj—percentage of patients diagnosed at a specific stage;
nj—number of patients at a specific stage;
N—total number of patients;
Ci—cumulative number of patients surviving up to period i;
nk—number of patients in period k.
In contrast, Stage III saw 55.26% of patients (42 out of 76) succumbing within one year:
and no patients survived beyond six years. For Stage IV, the situation was even more critical, with 78.13% (25 out of 32) of patients living less than one year:
and none survived beyond three years.
Patients diagnosed at earlier stages showed markedly better survival outcomes, highlighting the vital role of early detection. The cumulative percentage of patients with survival durations beyond five years in Stage II was 8.33% (8 out of 96):
Survival outcomes for patients with Stages III and IV cancers are poor, with the majority succumbing within one year. This highlights the urgent need for improved palliative care and targeted therapies to address advanced-stage cancers.
Additionally, cases with an undetermined cancer stage exhibit survival patterns similar to advanced-stage cancers. Of these 32 cases, 59.38% (19 patients) lived less than one year, and none survived beyond four years:
This similarity may reflect late diagnoses or limited diagnostic data, emphasizing the necessity for thorough and timely diagnostics.
This analysis highlights the importance of early detection and comprehensive care strategies for elderly cancer patients. Efforts should prioritize access to screenings, improved diagnostics, and stage-specific treatments to enhance survival.
Most long-lived cancer patients (146 cases, 46.79%) survived less than a year post-diagnosis. Survival durations fell into five categories: under one year, 1–2 years, 3–4 years, 5–6 years, and over six years (Figure 3). Only 11 patients (3.53%) survived beyond six years, indicating poor long-term outcomes.
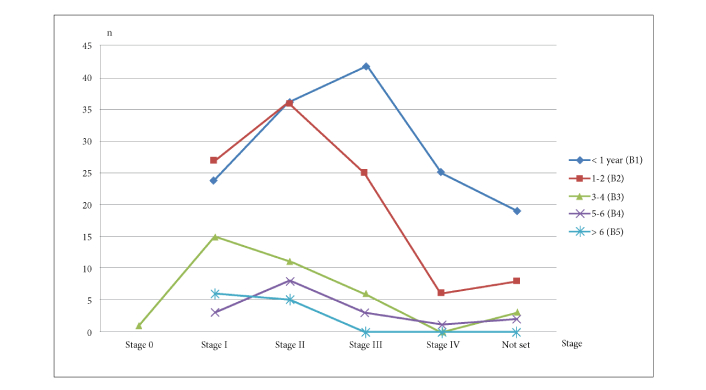
Survival duration of longlivers men with cancer based on the stage of their disease at diagnosis
In the 90–91 age group (144 patients, 46.15%), 66 (45.83%) survived less than a year, suggesting late-stage diagnosis. Only 6 (4.17%) lived beyond six years, reinforcing the poor prognosis even among younger long-lived individuals.
In the 92–93 age group (99 patients, 31.73%), 46 (46.46%) died within a year, and only 3 (3.03%) survived beyond six years, highlighting a steep survival decline with age. Among 46 patients (14.75%) aged 94–95, half (23, 50%) died within a year, with no survivors beyond six years, emphasizing the challenges of cancer treatment in this group. Of the 18 patients (5.77%) aged 96–97, 10 (55.56%) died within a year, with no survival beyond five years, indicating reduced treatment options and resilience with age. The smallest group, 98+ years (5 patients, 1.6%), had 2 deaths (40%) within a year and no survivors beyond three years, reflecting the extremely limited survival rates in this age group.
There is a strong inverse relationship between age and survival duration in long-lived cancer patients. Using Pearson’s correlation coefficient (r) to quantify this relationship, we find that r = −0.93, which indicates a very strong negative correlation between age and survival duration.
Age groups (X). The midpoints (Xi) of the age ranges are as follows:
90–91 years: X1 = 90.5;
92–93 years: X2 = 92.5;
94–95 years: X3 = 94.5;
96–97 years: X4 = 96.5;
Over 98 years: X5 = 99.0.
Survival duration (Y). The survival duration (Yi) represents the mean survival time for each age group. It is calculated as: mean survival time for each age group, calculated as:
where:
nj is the number of patients in the group;
T is the survival time within the interval;
N is the total number of patients.
The Pearson correlation coefficient (r) is calculated as follows:
where:
Xi and Yi are the midpoint and mean survival duration for each group;
Younger long-lived individuals (90–91 years) had better survival rates, with 11.11% living beyond 3–6 years. Survival duration decreases significantly with age due to:
Late-stage diagnosis (many cancers are detected at advanced stages).
Comorbidities and frailty (limiting treatment options and resilience).
Focused care (for patients over 96 years, curative treatment is often infeasible, necessitating palliative care).
Treating cancer in the oldest old is challenging due to these factors. In most patients across all age groups (146 cases, 46.79%) succumbed within one year, indicating late-stage diagnosis and limited treatment efficacy. Early diagnosis is crucial for improving outcomes, especially in the 90–91 age group. For those over 96, survival beyond one year is rare, with no patients surviving past six years, emphasizing the need for palliative care rather than curative treatment in this population.
The overall health of elderly patients is also particularly important. According to data from Kazaure et al. [9], primary healthcare is critical to improving treatment outcomes, as patients who undergo regular medical check-ups have a lower risk of postoperative complications and mortality. Health support factors, such as regular physical activity and proper nutrition, are also vital in improving treatment results.
Regarding specific types of cancer, one of the main concerns is prostate cancer in elderly men. The authors emphasize that prostate cancer in patients over 70 years old tends to be more aggressive, and treatment often leads to a deterioration in quality of life [10].
Among the most common types of cancer in older adults is lung cancer, which poses a serious threat to the health of the elderly. Scientists conducted a study showing that among individuals over 90 years of age who had lung cancer, survival rates are low, particularly due to late medical visits and limited options for surgical intervention [11].
In conclusion, cancer in men aged 90 and older presents an important issue in oncology. Treatment can be effective and well-tolerated; however, it requires a careful approach, taking into account the individual characteristics of each patient, particularly their overall health status. Further research should focus on optimizing treatment and ensuring better outcomes for this patient group. Age stands out as one of the most significant factors influencing cancer survival. A majority of the patients analyzed were aged 90–91 years, making up 46.15% of the sample. However, the number of cases dropped sharply in the older age groups (92–93, 94–95, 96–97, 98+ years). This decrease in cases correlates with a decline in survival rates as age increases, particularly in rural areas, where fewer patients survive past the 93-year mark. These observations emphasize the challenges older cancer patients face in terms of treatment efficacy and survival outcomes, especially in less urbanized regions where healthcare resources are often limited.
Patients over 90 years old with cancer often face low survival rates due to their age and comorbidities. Research indicates that survival rates for this age group are significantly lower compared to younger patients [11]. Aging is accompanied by numerous physiological changes that can reduce treatment effectiveness and recovery potential. In patients over 90, cancer is often diagnosed at advanced stages, which also decreases the chances of successful treatment [12]. Age is an important factor, but it is not the only one influencing therapy selection, as comorbidities and the functional state of patients can significantly affect treatment outcomes [11].
A significant body of research emphasizes the importance of an individualized approach to treating patients over 90, particularly when choosing therapeutic strategies for those with cancer [13]. For example, for elderly patients with non-small cell lung cancer (NSCLC), treatment significantly increases survival compared to those who do not receive therapy [11]. However, most patients over 90, due to health issues such as cardiovascular diseases and cognitive impairments, often do not receive the necessary treatment [12].
A recent study in the Lviv region of Ukraine analyzed cancer cases among long-lived women aged 90 and older, highlighting that while life expectancy was strongly correlated with cancer stage, it showed weak correlations with age at diagnosis and treatment type, suggesting that less aggressive treatments in this age group may contribute to poorer survival outcomes [14].
Thus, future research should focus on improving early detection methods for oncology diseases in elderly patients and developing individualized treatment approaches that take into account the physical and psychological changes characteristic of this age group.The finding that urban areas have a higher proportion of long-lived cancer patients (72.12%) compared to rural areas (27.88%) echoes conclusions found in multiple studies. For instance, found that urban areas tend to have better access to healthcare facilities, contributing to longer survival rates [15]. Similarly, note that advanced cancer treatments and technologies are often more accessible in urban environments, improving patient survival rates [16]. Rural patients, on the other hand, face challenges such as limited healthcare access and socio-economic barriers that can contribute to poorer outcomes, as highlighted in the study.
The analysis that cancer patients aged 90–92 years had the highest survival rates, while survival decreased sharply in older age groups, is consistent with findings in several studies. Emphasize that age significantly affects cancer survival, with survival rates diminishing in patients over 90 years old [11, 17]. The decline in survival in patients over 93 years, particularly in rural areas, aligns with doctors, who underscore the complexity of treating very old patients with multiple comorbidities, where the efficacy of cancer treatments is often limited [5].
The findings on the impact of cancer stages align with research, which emphasize that early-stage cancers are more treatable and associated with better survival rates [11, 18]. In contrast, researchers indicate that advanced-stage cancers, particularly Stage IV, are strongly linked to shortened survival durations [6, 19]. The lack of survivors beyond three years in patients with Stage IV cancer in the study mirrors the findings, which suggest that older patients diagnosed with late-stage cancers face the worst prognoses [20]. The stage of cancer at diagnosis is strongly correlated with survival duration. Early-stage cancers (Stage 0 and Stage I) were associated with longer survival times, with some patients surviving beyond five years. In contrast, advanced-stage cancers (Stage III and IV) were linked to significantly shorter survival times, particularly in patients over 90 years old. The majority of patients with Stage IV cancer succumbed within one year, and none survived beyond three years. This highlights the critical need for early detection and more aggressive treatment for patients diagnosed at earlier stages, as late-stage diagnoses drastically reduce survival chances.
The relationship between increasing age and shorter survival times, particularly after the age of 96, is well-supported in literature. Note that older cancer patients experience frailty and multiple comorbidities, which reduce their capacity to withstand aggressive treatments [4]. The results are consistent with the data that survival beyond one year is increasingly rare in patients aged 96 and older, highlighting the need for a more palliative approach in the oldest groups [16].
As age increases, survival rates tend to decrease significantly. Among the youngest age group (90–91 years), some patients survived beyond three years, suggesting better treatment outcomes and greater resilience. However, as patients aged, particularly those over 96 years, survival beyond one year became exceedingly rare. These findings underline the need for a shift in focus toward symptom management and palliative care in the oldest age groups, as aggressive treatments may offer little benefit due to frailty and comorbidities.
The study aligns with current research, confirming that urban residency, younger age, early cancer stages, and specific treatment types (e.g., surgery) significantly contribute to better cancer survival outcomes in elderly patients. It also highlights the challenges posed by advanced age, late-stage cancer, and limited healthcare access in rural areas. These findings contribute valuable insights to the growing body of literature on cancer survival in the elderly. Therefore, urbanization, age, and access to healthcare are critical factors influencing longevity among cancer patients. Addressing disparities in healthcare accessibility between urban and rural areas could improve outcomes for rural residents.
Our retrospective study included 312 long-lived men from Lviv region (Ukraine) aged over 90 diagnosed with 25 types of cancer during 1991–2019, the median of age was 92.5 ± 2 years.
Among the long-lived men, nearly a third were patients with skin cancer (30.77%), followed by prostate cancer in second place (19.87%), and stomach cancer in third place (7.69%). In the studied sample, there were 5 oncological diagnoses, each with 1 (0.32%) case (retroperitoneum, adrenal gland, non-follicular lymphoma, lymphoid leukemia and malignant neoplasm of other and ill-defined sites).
A higher proportion of long-lived cancer patients resided in urban areas (72.12%) compared to rural areas (27.88%), indicating that urban residents have better access to healthcare, diagnostics, and treatment options, which may contribute to improved survival rates.
A very strong negative correlation exists between age and survival duration in long-lived cancer patients, with Pearson's correlation coefficient (r) of −0.93. As age increases, the number of cancer diagnoses significantly declines, particularly after the age of 93.
Early stages of cancer (Stages 0 and I) showed significantly better survival outcomes, with a 100% survival rate for 3–4 years in Stage 0, while in Stage IV, 78.13% of patients succumbed within a year, and none survived beyond three years.
For patients over 96 years old, survival beyond one year is rare, highlighting the need for a palliative care approach focused on symptom management rather than curative treatment.
Future research should focus on improving early cancer detection methods for elderly patients and developing tailored treatment approaches that address the unique physical and psychological challenges of aging, as well as improving healthcare access for rural populations.
NK: Conceptualization, Writing—original draft, Writing—review & editing. ZD: Formal analysis, Visualization, Writing—original draft. YS: Methodology, Validation, Writing—original draft. OT: Methodology, Methodology. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
All authors have disclosed no conflicts of interest.
This study was approved by the Ethics Committee of the Lviv Oncologic Regional Treatment and Diagnostic Center, Ukraine (Approval No. 03/25-3, dated March 19, 2025).
Individual patient consent was not required, as the study was based on retrospective analysis of anonymized data collected for public health and epidemiological purposes. The registry contains no personally identifiable information, and all data were fully de-identified prior to researcher access. The institutional ethics committee confirmed that the use of such anonymized data does not pose any risk to individual privacy and complies with national and international ethical standards for research involving human subjects. Therefore, the requirement for informed consent from each patient was waived.
Not applicable.
All data generated or analysed during the present study are available from the corresponding author on reasonable request.
This study received no specific grant from any funding agency in the public, commercial, or notfor-profit sectors.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1234
Download: 21
Times Cited: 0
