Affiliation:
1Doctoral Program of Biomedicine, Faculty of Medicine, Universitas Andalas, Padang 25163, Indonesia
2Department of Internal Medicine, Faculty of Medicine, Universitas Riau, Pekanbaru 28131, Indonesia
ORCID: https://orcid.org/0000-0001-6645-9023
Affiliation:
2Department of Internal Medicine, Faculty of Medicine, Universitas Riau, Pekanbaru 28131, Indonesia
ORCID: https://orcid.org/0000-0001-8680-7865
Affiliation:
2Department of Internal Medicine, Faculty of Medicine, Universitas Riau, Pekanbaru 28131, Indonesia
ORCID: https://orcid.org/0009-0004-6983-0659
Affiliation:
2Department of Internal Medicine, Faculty of Medicine, Universitas Riau, Pekanbaru 28131, Indonesia
ORCID: https://orcid.org/0009-0006-0434-7708
Affiliation:
2Department of Internal Medicine, Faculty of Medicine, Universitas Riau, Pekanbaru 28131, Indonesia
ORCID: https://orcid.org/0009-0001-1713-6970
Affiliation:
2Department of Internal Medicine, Faculty of Medicine, Universitas Riau, Pekanbaru 28131, Indonesia
ORCID: https://orcid.org/0000-0001-7435-6550
Affiliation:
3Faculty of Medicine and Health Sciences, Universitas Bangka Belitung, Pangkalpinang 33215, Indonesia
ORCID: https://orcid.org/0009-0008-8702-9362
Affiliation:
4Department of Public Health, Faculty of Medicine, Universitas Riau, Pekanbaru 28131, Indonesia
ORCID: https://orcid.org/0000-0001-7659-6689
Affiliation:
5Eijkman Research Center of Molecular Biology, National Research and Innovation Agency (BRIN), Cibinong 16911, Indonesia
ORCID: https://orcid.org/0000-0003-0097-2435
Affiliation:
5Eijkman Research Center of Molecular Biology, National Research and Innovation Agency (BRIN), Cibinong 16911, Indonesia
ORCID: https://orcid.org/0000-0002-7639-7441
Affiliation:
6Department of Pharmacology, Faculty of Medicine, Universitas Andalas, Padang 25163, Indonesia
ORCID: https://orcid.org/0000-0001-5743-3331
Affiliation:
7Department of Radiology, Division of Nuclear Medicine, Faculty of Medicine, Universitas Andalas, Padang 25163, Indonesia
Email: aelliyanti@med.unand.ac.id
ORCID: https://orcid.org/0000-0003-0812-8052
Explor Med. 2025;6:1001354 DOl: https://doi.org/10.37349/emed.2025.1001354
Received: May 01, 2025 Accepted: July 21, 2025 Published: September 08, 2025
Academic Editor: Anna Maria Spagnolo, University of Genova, Italy
Background: Streptococcus pneumoniae is a leading cause of respiratory infections and invasive disease. Although its burden in children is well-known, adult asymptomatic carriage remains under-investigated, particularly in tropical regions.
Methods: This meta-analysis, registered in PROSPERO (CRD420244559641) and adhering to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines, searched PubMed, ScienceDirect, and Epistemonikos for studies published in the past decade. Eligible studies on adult carriage were included in the PECOS framework. Data were independently extracted by multiple reviewers and pooled using a random-effects meta-analysis. Subgroup analyses evaluated differences by sex, comorbidities [e.g., human immunodeficiency virus (HIV), diabetes], and behavioral factors (e.g., contact with children). Geospatial variations were mapped using RStudio and ggplot2, and study quality was appraised via the Joanna Briggs Institute tools.
Results: Thirty-two studies (n = 56,409) revealed a pooled carriage prevalence of 17% (95% CI: 0.12–0.23). Carriage was higher in females (57% vs. 47% in males) and in HIV-positive individuals (78%), while type 2 diabetes was linked to lower carriage (6%). Elevated rates were mapped in Gambia, Fiji, and Malawi. Non-vaccine serotypes (68%) predominated over vaccine serotypes (32%).
Discussion: Adult S. pneumoniae carriage is globally significant and heterogeneous, underscoring the need for targeted surveillance and vaccination strategies, especially in tropical, high-density settings.
Streptococcus pneumoniae (S. pneumoniae; also known as pneumococcus) poses a huge burden on the health system due to a high number of invasive pneumococcal disease (IPD) costs and mortality rates, especially in groups considered at risk, namely children below 5 years, the elderly, and the adult group with comorbidity [1]. A global study in 2016 by Global Burden of Disease (GBD) showed S. pneumoniae to be the global leading cause of lower respiratory infection morbidity and mortality, with an incidence of 26.7 per 1,000 people and total deaths of 16.1 per 100,000 people worldwide [2]. Aside from being one of the most common causes of respiratory tract infections in children, studies have shown that S. pneumoniae is also the most common cause of community-acquired pneumonia, causing acute respiratory failure in adults. Adults tend to be more commonly infected by S. pneumoniae than children (72.8 vs. 70.7 per 1,000 population), and it is more commonly a cause of death in adults than in children (122.3 vs. 54 per 100,000 population) [2]. The mortality rate is approximately 25–30% if accompanied by bacteremia, with variations depending on the onset, disease severity, and host factors [3]. Elevated mortality rates due to S. pneumoniae infections are standard, especially in adults with pre-existing pulmonary conditions such as chronic obstructive pulmonary disease (COPD) or in immunocompromised individuals [4, 5]. Following this, despite the broad adoption of pneumococcal conjugate vaccination, the overall mortality rate from IPD has remained high, at 20.8% [6].
Commonly, S. pneumoniae is a commensal bacterium on the mucosal surface of the upper airways that enables transmission from person to person. One of the notable characteristics of S. pneumoniae is its ability to infect and colonize asymptomatically in a significant number of adult populations. Studies show that up to 5–10% of adults’ nasopharynx may be asymptomatically colonized by S. pneumoniae, which could be a potential source of community-acquired pneumonia [7, 8]. Approximately 20–40% of the healthy child population asymptomatically carry S. pneumoniae orally and in their nasopharynx [9]. This asymptomatic colonization plays an essential role in person-to-person transmission, especially in young children who are the primary transmission source to adult populations with the highest carriage rates (40–60%) compared to adults, with carriage rates only at 3–4% [10].
Understanding the prevalence of S. pneumoniae carriage, especially in adults, is essential for predicting the risk of transmission and acquisition. Adult patients with S. pneumoniae carriage tend to have additional comorbid diseases and other factors, such as immunocompromised states, additional habits and lifestyles, which could lead to higher mortality rates [11, 12]. Prevalence of S. pneumoniae carriage is limited. This study aims to analyze the prevalence in adults (ages 18–65) to assess its impact on transmission carriage and potential invasive disease.
The study’s protocol, which includes information about the literature search approach, has been published and registered in PROSPERO under the registration number CRD420244559641. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses was followed in the writing of this study [13].
Various databases were used, including PubMed, ScienceDirect, and Epistemonikos. The following are the inclusion criteria: (1) articles written in English or Indonesian, (2) appropriate for the PECOS framework, and (3) published within the last ten years. The population must be between the ages of 18 and 65, and the review, advertisement, or case report design must be included. A combination of keywords such as “Streptococcus pneumoniae carriage OR Pneumonia carrier OR Pneumococcal carriage” was utilized during the literature searches.
The PECOS framework was used to select the studies: observational study (cross-sectional/cohort), clinical trial, or RCT (study); patients between ages of 18 and 65 years; screening for S. pneumoniae carriage (exposure); no comparison restriction (comparison); and incidence/number of individuals with S. pneumoniae colonization (outcome). A person with S. pneumoniae carriage does not exhibit any clinical symptoms, but nasopharyngeal swab or serological testing reveals signs of S. pneumoniae infection or colonization [14].
The data were independently extracted by seven investigators (DR, AMS, NCE, RTP, ZERN, A Elisabet, and FG) as per the PRISMA process, and any disagreements were resolved through discussion with the other authors. Various data were extracted from included articles, such as first author, year of publication, country, study design, total sample, number of incidences, gender, co-infection with human immunodeficiency virus (HIV), number of patients with diabetes, history of smoking, history of contact with children confirmed with S. pneumoniae, and serotype of S. pneumoniae based on pneumococcal conjugate vaccine (PCV)13 coverage.
We used RStudio (2024.09.0+375) for the proportion meta-analysis and geospatial analysis. Proportional analysis used the library (meta) with the metaprop program code. Proportional analyses were performed on case incidence as well as data such as smoking history, gender, contact with children, and comorbidities (HIV and diabetes mellitus). Incidence with a total incidence ratio of 1:1 was not included because it would be confounding. Data were presented using a forest plot with proportion values and 95% CI. Results were considered accurate when the 95% CI value narrowed and less accurate when the 95% CI value moved away from the proportion value. Heterogeneity tests were also conducted by displaying the I2 and tau-squared (τ2) values. The higher the I2 value, the higher the heterogeneity between studies. The proportion value was carried out with the random effect model approach so that the results can represent general conclusions (not only limited to research). We also performed the publication bias analysis in RStudio and presented the data using a funnel plot. Analysis of the plot assessed the visual symmetry to determine if there was publication bias.
We also used the same RStudio application using the ggplot2 package for geospatial analysis. Data were mapped using the incidence rate (IR) with the equation:
The data will be presented using a world map where the proportions are based on color gradations; the higher the proportion of incidence, the more intense the color.
The risk of bias for each included article was checked with the Joanna Briggs Institute (JBI) critical appraisal tools based on each study design. The JBI critical appraisal tool was chosen because it has a wide range of study design flexibility that can be used. This tool uses a questionnaire with yes (Y) or no (N) answers. Each author assesses each article and gets a score with a formula: total number of correct/total number of questions. The data are presented with a percentage score and displayed in a bar chart to see the average value of each article. JBI sets no value limit in determining low, medium, or high bias risk. Therefore, in this study, it was agreed that a presentation value below 50% is referred to as “High Risk”, a value of 50–75% is referred to as “Medium Risk”, and above 75% is referred to as “Low Risk”. We also analyzed publication bias using a funnel plot with trim and fill imputed studies.
A comprehensive search across multiple databases identified 97,036 articles, of which 395 were removed due to duplication. Duplicates were initially detected using the reference manager Zotero, followed by a manual review for databases where automated duplication detection was not feasible. After the removal of duplicates, 96,641 articles remained for screening. Of these, 96,412 were excluded for failing to meet the eligibility criteria. Additionally, 103 articles were inaccessible as they only provided abstracts or lacked full-text availability.
Following this process, 126 articles underwent further assessment. Among these, 74 articles were excluded because the subjects were not between 18 and 65 years of age. Notably, age-related exclusions could not be determined solely based on the title, as many studies encompassed a broad population ranging from children to the elderly. Since extracting adult-specific data was not feasible, exclusion was the most appropriate approach to minimize bias, ensuring alignment with the study’s focus on adult populations. Consequently, 32 articles were retained for final review. The entire study selection process is illustrated in Figure 1.
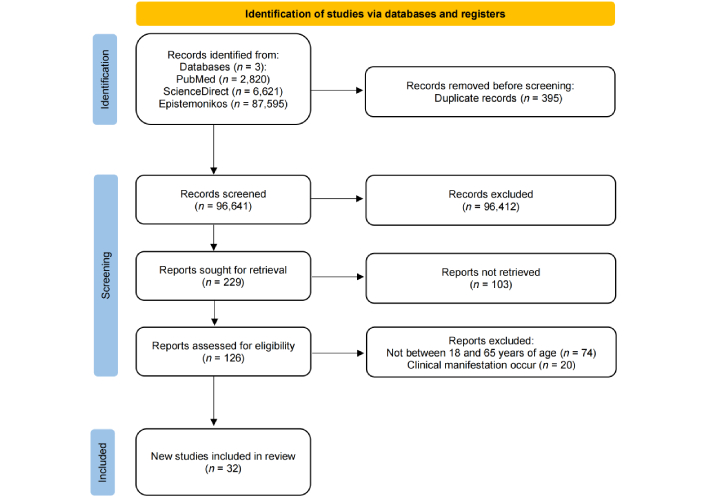
PRISMA flow chart for screening and selection of articles. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses. Adapted from [13]. © 2021, The Author(s). Distributed under a Creative Commons Attribution license (CC BY 4.0).
The cross-sectional and cohort research designs dominated this study because they are suitable for tracing the magnitude of cases that occur. The studies reported are from various countries, and to visualize the reports and magnitude of cases from each country, as documented in this study, they are discussed in the geospatial analysis. Characteristics of all included studies are compiled in Table 1.
Characteristics of included studies.
| No | Author, year | Design | Country | Total sample | Number of incidence |
|---|---|---|---|---|---|
| 1 | Swarthout et al. [15] 2020 | Cohort | Malawi | 1,770 | 714 |
| 2 | Ngamprasertchai et al. [16] 2023 | CS | Thailand | 25 | 10 |
| 3 | Reta and Daka [17] 2023 | CS | Ethiopia | 323 | 41 |
| 4 | Steurer et al. [18] 2022 | CS | Austria | 437 | 21 |
| 5 | Parker et al. [19] 2023 | CS | USA | 1,278 | 117 |
| 6 | Petrović et al. [20] 2022 | Cohort | Serbia | 70 | 2 |
| 7 | Thindwa et al. [21] 2022 | CS | Malawi | 2,067 | 784 |
| 8 | Harimurti et al. [22] 2021 | CS | Indonesia | 557 | 59 |
| 9 | Lomardo et al. [23] 2021 | CS | Brazil | 265 | 18 |
| 10 | Ly et al. [24] 2021 | CS | France | 507 | 60 |
| 11 | Thindwa et al. [25] 2021 | Cohort | South Africa | 726 | 266 |
| 12 | Neal et al. [26] 2020 | CS | Fiji | 2,035 | 193 |
| 13 | Amritha et al. [27] 2020 | CS | India | 400 | 74 |
| 14 | Adler et al. [28] 2019 | CS | UK | 795 | 52 |
| 15 | de Steenhuijsen Piters et al. [29] 2019 | Cohort | UK | 451 | 117 |
| 16 | Roca-Oporto et al. [30] 2019 | Cohort | Spain | 500 | 39 |
| 17 | Sutcliffe et al. [31] 2019 | CS | USA | 509 | 74 |
| 18 | Yahiaoui et al. [32] 2018 | CS | Austria, Belgium, Croatia, France, Hungary, Spain, Sweden, Netherlands, UK | 32,161 | 937 |
| 19 | Xie et al. [33] 2018 | CS | China | 173 | 97 |
| 20 | Heinsbroek et al. [34] 2018 | CS | Malawi | 418 | 64 |
| 21 | Usuf et al. [35] 2018 | Cohort | Western Gambia | 374 | 91 |
| 22 | Tramuto et al. [36] 2017 | CS | Italy | 80 | 46 |
| 23 | Kolšek-Šušteršič et al. [37] 2017 | CS | Slovenia | 38 | 3 |
| 24 | Conklin et al. [38] 2016 | CS | Kenya | 973 | 375 |
| 25 | Bosch et al. [39] 2016 | CS | Netherlands | 322 | 31 |
| 26 | Harimurti et al. [40] 2016 | CS | Indonesia | 200 | 20 |
| 27 | Wyllie et al. [41] 2016 | CS | Netherlands | 621 | 36 |
| 28 | Benkouiten et al. [42] 2014 | Cohort | KSA | 129 | 63 |
| 29 | Bos et al. [43] 2014 | CS | Mozambique | 177 | 41 |
| 30 | Mosser et al. [44] 2014 | Cohort | USA | 440 | 285 |
| 31 | Reisman et al. [45] 2014 | CS | USA | 5,975 | 898 |
| 32 | Memish et al. [46] 2015 | CS | KSA | 1,613 | 121 |
CS: cross-sectional.
The incidence data, involving 56,409 adults, were pooled using a statistical proportional meta-analysis, as presented in Figure 2. The analysis revealed that 17 out of 100 adults carried S. pneumoniae (95% CI: 0.12–0.23). Heterogeneity, assessed using the inconsistency index (I2), was found to be 99.5%, indicating substantial variability among the included studies. Additionally, the τ2 value was 1.1800, suggesting a high degree of heterogeneity. This finding was further supported by a statistically significant P-value < 0.0001.
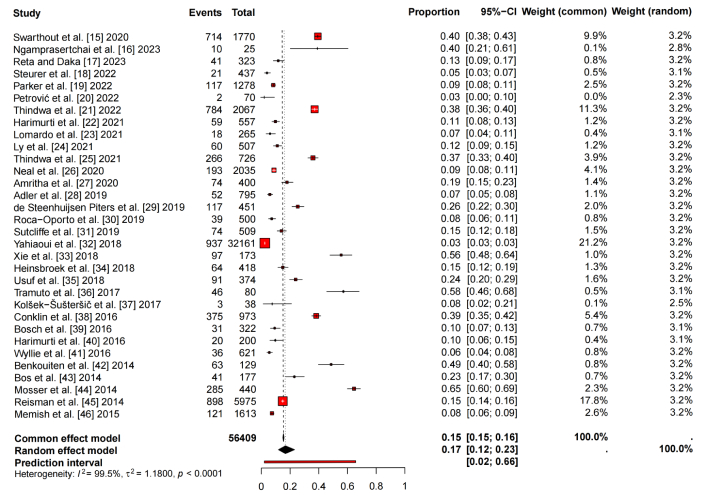
Total prevalence of Streptococcus pneumoniae carriage by proportional meta-analysis. τ2: tau-squared.
The proportional meta-analysis revealed significant variations in S. pneumoniae carriage across different demographic and clinical subgroups of adults, emphasizing the influence of host factors on colonization dynamics. The carriage rate was found to be lower in males (47%; 95% CI: 0.35–0.59; Figure 3A) compared to females (57%; 95% CI: 0.40–0.72; Figure 3B), suggesting potential sex-related differences in susceptibility or exposure patterns. This discrepancy may be attributed to variations in immune response, behavioral factors, or healthcare-seeking tendencies, all of which warrant further investigation.
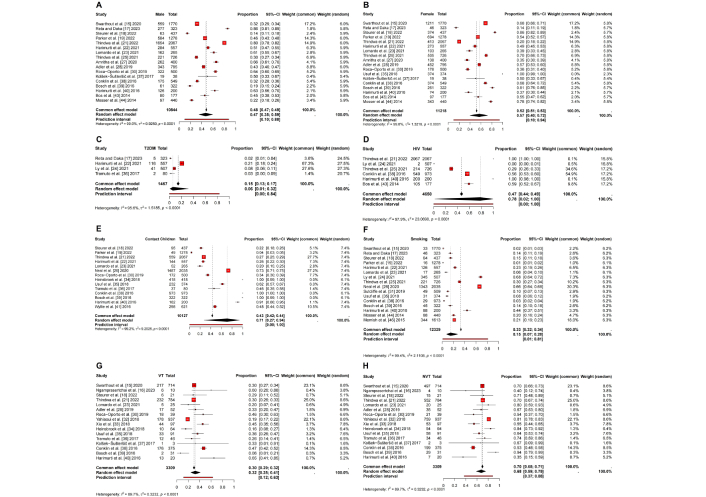
Pooling proportion meta-analysis with various other variables from Streptococcus pneumoniae carriage patients. (A) Pooling proportion meta-analysis of male patients; (B) pooling proportion meta-analysis of female patients; (C) pooling proportion meta-analysis of patients with type 2 diabetes mellitus (T2DM); (D) pooling proportion meta-analysis of patients with HIV; (E) pooling proportion meta-analysis of patients with history of contacting children < 5 years; (F) pooling proportion meta-analysis of patients with history of smoking; (G) pooling proportion meta-analysis of proportion of Streptococcus pneumoniae carriage vaccine type; (H) pooling proportion meta-analysis of proportion of Streptococcus pneumoniae carriage non-vaccine type. HIV: human immunodeficiency virus; τ2: tau-squared; VT: vaccine type; NVT: non-vaccine type.
Among individuals with underlying medical conditions, those with type 2 diabetes mellitus (T2DM) (6%; 95% CI: 0.01–0.32; Figure 3C) exhibited a substantially lower carriage rate than the general population. This finding aligns with previous research indicating that hyperglycemia and metabolic dysregulation in diabetes may alter mucosal immunity, potentially influencing pneumococcal colonization. In contrast, the highest carriage rate was observed in individuals living with HIV (78%; 95% CI: 0.02–1.00; Figure 3D), reflecting the well-established association between immunosuppression and increased bacterial colonization. The wide confidence interval in this subgroup suggests variability across study populations, likely due to differences in antiretroviral therapy coverage and immune status. Given the heightened risk of IPD in immunocompromised individuals, this finding underscores the critical need for targeted vaccination and preventive strategies.
Environmental and behavioral factors also played a significant role in pneumococcal carriage. Individuals with a history of contact with children (71%; 95% CI: 0.27–0.94; Figure 3E) exhibited a higher carriage rate, reinforcing the well-documented role of young children as primary reservoirs for pneumococcal transmission. Close interactions, particularly within household settings, facilitate bacterial exchange, which is of particular concern for adults in caregiving roles. Similarly, a history of smoking (15%; 95% CI: 0.07–0.28; Figure 3F) was associated with a lower yet notable carriage prevalence. Tobacco smoke is known to impair mucosal immunity and disrupt the respiratory microbiome, which may either promote or inhibit pneumococcal colonization depending on the stage of infection and individual susceptibility.
Lomardo et al. [23] (2021) reported that 18 subjects carried pneumococcus among 265 adults. They found that multiple colonization with two distinct serogroups/serotypes was confirmed in seven subjects, providing clear evidence of multi-serotype carriage within the same individual [23]. Analysis of serotype distribution showed that vaccine type (VT) strains accounted for 32% (95% CI: 0.25–0.41; Figure 3G), whereas non-vaccine type (NVT) strains were more prevalent at 68% (95% CI: 0.59–0.75; Figure 3H). This finding underscores the potential limitations of current pneumococcal vaccination programs, as NVTs appear to be more prevalent in colonized adults. The phenomenon of serotype replacement, wherein vaccine-targeted strains decline while non-vaccine strains expand, has been observed following widespread PCV implementation. This shift highlights the importance of ongoing vaccine surveillance. It suggests a need for broader-spectrum or next-generation vaccines to protect against S. pneumoniae colonization and subsequent invasive disease effectively.
These findings illustrate the heterogeneity of S. pneumoniae carriage across different populations, emphasizing the interplay between demographic, clinical, and environmental factors. The high carriage rate observed in immunocompromised individuals and those with frequent child contact suggests that these groups should be prioritized for targeted interventions. Additionally, the predominance of NVTs highlights an urgent need for enhanced surveillance and serotype shifting analysis.
Figure 4 presents the geospatial for global IR per 100,000 population of S. pneumoniae carriage across included studies, classified into six categories: very low (< 0.05), low (0.05–0.20), moderate (0.20–1.00), high (1.00–10.00), very high (≥ 10.00), and not available. Based on the geospatial analysis standardized per 100,000 population, the incidence of S. pneumoniae carriage varied across regions. Globally, the highest IR were observed in Gambia (IR 57.42), Fiji (IR 21.1), and Malawi (IR 8.21). That Gambia and Malawi have the highest IR may be associated with limited health infrastructure, poor access to healthcare services, and high HIV prevalence. In Fiji, a high incidence was also observed, which may be linked to lower healthcare capacity and frequent close contact with children under five years of age.
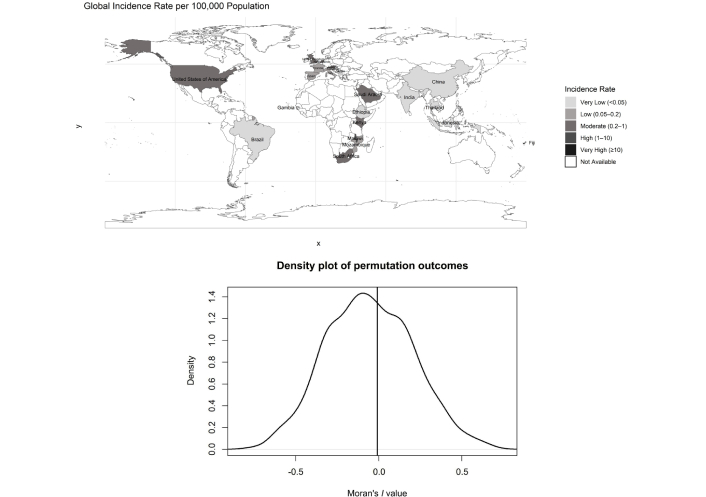
Geospatial analysis for global incidence rate per 100,000 population of Streptococcus pneumoniae carriage summarized in GeoMap with ggplot2 package [47].
Asian countries such as Thailand, India, and China showed similar and relatively very low IRs (IR 0.01). In Europe, countries demonstrated varied patterns, but generally fell within the low to moderate range, with the United Kingdom reporting an IR of 0.25, the Netherlands IR 0.39, Austria IR 0.24, and the lowest observed in Slovenia with an IR of 0.01, potentially reflecting the impact of robust vaccination programs and effective public health interventions.
Within Africa, incidence varied widely, with Gambia and Malawi at the highest end, while South Africa (IR 0.45) had notably lower rates. This disparity may be partly explained by differences in national economic status, as South Africa is classified as an upper-middle-income country, whereas Gambia and Malawi are categorized as low-income countries.
However, the observed heterogeneity in carriage rates across continents suggests that multiple factors—including socioeconomic status, healthcare accessibility, and pneumococcal serotype distribution—may play crucial roles in shaping regional epidemiology.
The density plot illustrates the null distribution of Moran’s I values generated through Monte-Carlo simulations, representing the expected outcomes under spatial randomness. The peak, which occurs slightly before Moran’s I = 0, highlights the central assumption of no spatial autocorrelation. The near-symmetric shape of the curve suggests that both clustering (positive Moran’s I) and dispersion (negative Moran’s I) are equally unlikely under the null hypothesis. The position of the observed Moran’s I, relative to this distribution, determines the significance of spatial autocorrelation. If the observed value lies in the tails of the distribution, it would indicate significant spatial clustering or dispersion. This density plot provides a robust statistical foundation for assessing the spatial dependency in the data and supports the validation of Moran’s I significance testing.
Based on the risk of bias assessment using the JBI critical appraisal tools (Figure 5), we found that the average quality score of all included studies was 83.65%. Overall, the included articles had a low risk of bias. However, when looking at the studies one by one, there are four studies that score between 50–75%, namely Thindwa et al. [25] 2021, Amritha et al. [27] 2020, Roca-Oporto et al. [30] 2019, and Xie et al. [33] 2018. The article cannot answer JBI tools in the form of confounding variables that are not mentioned and strategies for overcoming these confounding variables. Some of them also have inclusion criteria that are less clear/convincing, so they are stated as unclear, and some do not ensure the validity and reliability of the exposure measurement.
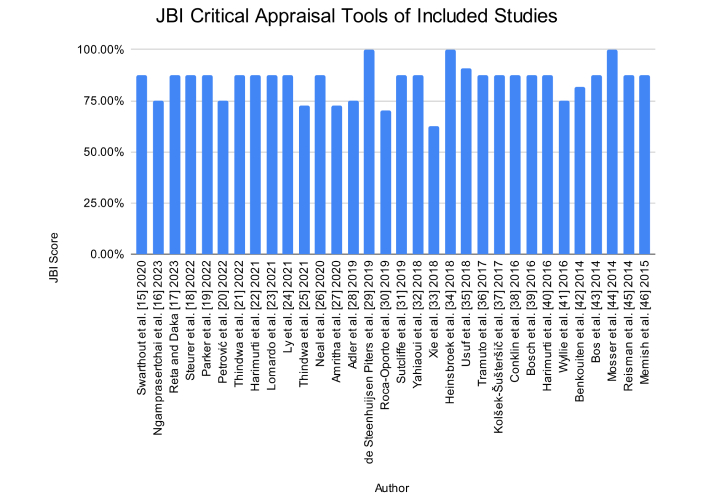
Scores of Joanna Briggs Institute (JBI) for all included studies using the JBI critical appraisal tools [48].
We analyzed publication bias using a funnel plot with trim and fill imputed studies. From Figure 6, we can visually see that there is asymmetry in the number of studies on the left side, especially at the bottom. This is indicative of a publication bias (file-drawer problem) where small studies that have no effect are less likely to be published.
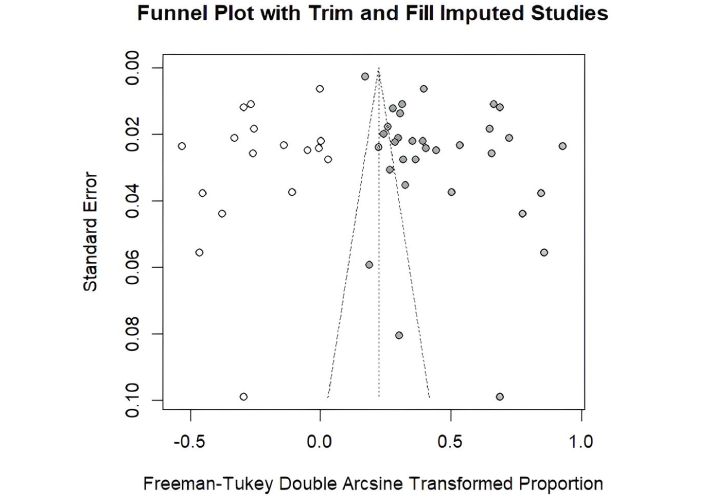
Funnel plot of all included studies using RStudio (https://posit.co/products/open-source/rstudio/). The white dots represent the original studies included in the meta-analysis. The filled (grey) dots indicate the imputed studies added by the trim-and-fill method to adjust for potential publication bias. The dots with stripes denote the adjusted pooled estimate after imputation.
This study utilized a population of 56,409, which showed a prevalence of S. pneumoniae carriage in adults of 17% globally (Figure 2). This indicates a considerable prevalence of adult carriage. This has two implications: (1) transmission of S. pneumoniae can occur between adults, and, therefore, raising awareness among this population is essential; (2) adults with immunocompromised states (e.g., HIV, diabetes mellitus) who are colonized with S. pneumoniae are at increased risk of developing IPD. These individuals may also serve as a source of transmission to others and could develop symptomatic disease themselves, as colonization may precede IPD [2]. Thus, this supports the urgency for the implementation of PCV research and vaccination in adults. The implementation of PCV vaccination in adults remains limited in many countries, despite the WHO having recommended PCV for specific high-risk groups. PCV administration in adults is expected to reduce the risk of carriage progressing to IPD. According to a meta-analysis of RCTs, the total combination vaccination effectiveness against IPD caused by any serotype was 63% (95% CI = 10–92%) [49]. Therefore, the data we have found on the magnitude of carriage cases should support the urgency of implementing PCV vaccination and development in adults.
We shared the prevalence of S. pneumoniae carriage for VT and NVT strains based on PCV13 coverage. The prevalence of VT strains accounted for 32% (95% CI: 0.25–0.41; Figure 3G), whereas NVT strains were more prevalent at 68% (95% CI: 0.59–0.75; Figure 3H). The five most frequent NVT serotype distributions as revealed by the previous studies were 19B, 18B, NT (non-typable), 24, and 19C. We suspected that there may be an influence of countries that have implemented the PCV in adults, resulting in a shift in the serotype of S. pneumoniae carriage. Therefore, country-specific widespread epidemiologic studies are needed to see the development of these carriage cases to be handled as effectively as possible according to epidemiology. Therefore, the implementation of PCV types determined based on the serotype distribution of the country will have a more effective outcome.
The results of geospatial analysis standardized per 100,000 population show that the incidence of S. pneumoniae carriage is still dominated by low-income countries (Gambia and Malawi), but Fiji, as an upper-middle-income country, also had a high incidence. This is very likely to allow contact between the carriage and other people so that the spread of cases is also high. Some countries show moderate IR, such as Saudi Arabia, Kenya, South Africa, the United States of America, the Netherlands, the United Kingdom, and Austria. Further analysis with Moran’s I shows that there is a significant spatial distribution, indicating a regional influence on incidence with the most likely variable being population density. The data imply that carriage cases may continue to circulate in the area, and if the person has good immunity, it may not be symptomatic, but if there are special conditions, it has the potential to develop into IPD and death. Therefore, every country that has a high population density needs to evaluate the carriage conditions and, if possible, properly implement the use of vaccinations.
We attempted to further analyze all articles that we were able to include, both sociodemographic variables and risk factors that might influence them. Based on gender, the proportion of incidence in women was higher than in men (57% vs. 47%). This finding aligns with earlier studies that demonstrate a greater susceptibility of females to upper respiratory tract infections, while males are more prone to infections in the lower respiratory tract. One biological factor contributing to the higher susceptibility of women to S. pneumoniae carriage is hormonal differences. Higher levels of estrogens in females have been shown to boost immune responses, particularly in the upper respiratory system, which may increase the chances of colonization by pathogens such as S. pneumoniae. Conversely, androgens, which are more common in males, tend to have anti-inflammatory effects, potentially leading to lower carriage rates in this group. Moreover, differences in immune system function between the two sexes may also lead to differences in the transmission of S. pneumoniae in men and women. Studies show that females typically demonstrate a more robust immune response to respiratory infections, which can promote bacterial colonization. This heightened immune response not only increases the chances of carrying S. pneumoniae but may also influence the severity of later infections [50].
We also explored other risk factors such as T2DM, HIV, and smoking history (Figure 3). The prevalence of carriage in HIV patients was higher among others, followed by smoking history and T2DM (78%, 15%, and 6% of the study population, respectively). This suggests HIV may be a factor in the high incidence of carriage. The most likely explanation is that the patient’s immunocompromised condition causes S. pneumoniae clearance to be ineffective and can actually thrive and infect others. Therefore, patients with HIV should be further monitored for the possibility of S. pneumoniae carriage and the condition is very likely to develop into IPD. This is in line with epidemiological research conducted by Carrim et al. [51] (2023) that patients with HIV have a high incidence of colonization and have a higher pneumococcal load than non-HIV patients.
One factor that also deserves attention is the history of contact with carriage children; the incidence of carriage in adults with a history of such contact is 71%. This is very logical because many studies to date have seen that the incidence of carriage is high in children, so PCV vaccination is an urgent need to prevent further pneumonia. Apparently, the parents of children are also victims and can be infected as well. Of particular concern is whether the child’s parents have conditions that increase the risk of progression from carriage to IPD. Therefore, holistic research is needed to look not only at the incidence of children, but to trace whether there is an adult with IPD, as there may be carriage in the house and it may come from the child, so that it can also be treated in the future. This becomes a holistic therapy to prevent recurrent infections.
This study showed a high overall heterogeneity (Figure 2) with an I2 of 99.5%. Many factors strongly influence this heterogeneity, such as country differences and population density, vaccination (PCV) implementation, weather that may affect transmission and immunity, and many others. Several questions were not answered in this study, such as whether the proportion was associated with the vaccination history of patients or their children. This could change the prevalence of the total case or the serotype of S. pneumoniae carriage. No information was found related to patients’ history of vaccination, due to PCV not being mandatory in several countries for adults. Therefore, it’s potentially the cause of the lack of data. Geospatial visualization is also limited based on published data; there may be epidemiological data that have not been published, which also affects the results of this research.
The strength of this study lies in showing that the magnitude of carriage in adults needs to be a concern, and the implementation of PCV in adults needs to be further evaluated and researched based on the distribution of serotypes in the region. This study also successfully showed that a history of contact with children and HIV may be a risk factor for the high incidence of carriage in adults. If adults have special conditions, it will lead to further disease progression to IPD, so that morbidity and mortality due to S. pneumoniae will worsen in the future. Country-specific epidemiologic studies need to be the focus, so that the country’s carriage problem, serotypes, and possible influencing factors can be studied to implement PCV vaccination in adults to reduce morbidity and mortality due to S. pneumoniae.
Globally involving 56,409 adults, the incidence of S. pneumoniae carriage was found to be 17%, with the incidence being more common in women (57%), patients with HIV (78%), those with a history of contact with carriage children (71%), and NVTs (68%). There is a tendency for spatial factors to influence prevalence rates, as evidenced by Moran’s I results. This study highlights the high prevalence of carriage in low-income countries such as Gambia and Malawi, emphasizing the need for targeted public health interventions in resource-limited settings. Due to the high morbidity and mortality of S. pneumoniae cases, PCV vaccination may be a solution to treat cases before they worsen.
HIV: human immunodeficiency virus
IPD: invasive pneumococcal disease
IR: incidence rate
JBI: Joanna Briggs Institute
NVT: non-vaccine type
PCV: pneumococcal conjugate vaccine
PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses
S. pneumoniae: Streptococcus pneumoniae
T2DM: type 2 diabetes mellitus
VT: vaccine type
τ2: tau-squared
Dani Rosdiana thanks LPDP – Indonesian Endowment Fund for Education Agency – Kemenkeu for the support.
DR: Conceptualization, Methodology, Investigation, Writing—original draft, Writing—review & editing. AMS: Methodology, Investigation, Formal analysis, Visualization, Writing—original draft, Writing—review & editing. NCE, RTP, ZERN, A Elisabet, and FG: Methodology, Investigation, Writing—original draft. SS: Formal analysis, Writing—original draft. RS, DS, CI, and A Elliyanti: Conceptualization, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The primary data for this meta-analysis were sourced online from databases listed in the methods. Referenced articles are accessible on PubMed, ScienceDirect, and Epistemonikos. All data are available from the corresponding author on reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1183
Download: 44
Times Cited: 0
