Affiliation:
Brain Tumor Molecular Medicine, Sheba Medical Center, Ramat Gan 52620, Israel
Email: Rutyshai@gmail.com
ORCID: https://orcid.org/0000-0003-3961-0693
Explor Med. 2025;6:1001335 DOl: https://doi.org/10.37349/emed.2025.1001335
Received: January 15, 2025 Accepted: May 08, 2025 Published: June 20, 2025
Academic Editor: Lindsay A. Farrer, Boston University School of Medicine, USA
The article belongs to the special issue Personalized Medicine in Cancer Therapy
The interplay between the gut microbiota and the central nervous system is increasingly recognized as a critical factor in the pathogenesis and treatment responsiveness of brain tumors. The brain interacts with microbial communities, both systemically through the gut-brain axis and locally within the tumor microenvironment. The gut microbiota regulates systemic immunity and modulates key processes such as blood-brain barrier integrity, cytokine signaling, and neuroinflammation—all of which influence glioma development and resistance to therapies. Evidence from preclinical models indicates that modulation of the gut microbiota can enhance anti-tumor immunity and improve responses to immune checkpoint inhibitors (ICIs). In parallel, recent discoveries reveal the presence of bacterial DNA and viable microbes within glioma tissue initiating signaling cascades that modulate immune cell recruitment and polarization. These microbial-immune interactions shape the tumor’s immune landscape, favoring either anti-tumor immunity or immune evasion depending on the context. Additionally, microbial-derived metabolites, such as short-chain fatty acids, have been shown to influence gene expression through epigenetic mechanisms, including histone acetylation and regulation by non-coding RNAs. Such effects may contribute to tumor cell plasticity, metabolic reprogramming, and resistance to therapy. The reciprocal influence of glioma and its treatment on gut microbial ecology is also an important consideration. Therapeutic interventions such as antibiotics, corticosteroids, and chemotherapy can significantly disrupt the gut microbiota, potentially diminishing the efficacy of microbiota-driven immunomodulation. Therefore, understanding the bidirectional dynamics of the gut-brain-tumor axis is essential for the development of microbiome-informed therapies. Despite these promising insights, several challenges remain. In this review, we synthesize current knowledge on the role of the gut and intratumoral microbiota in glioma biology and treatment, focusing on immune modulation, therapeutic responsiveness, and potential for microbiota-informed interventions. We also discuss existing controversies, methodological limitations, and future research priorities in the context of advancing microbiome-based strategies in neuro-oncology.
Gliomas, particularly glioblastoma (GB), represent some of the most aggressive and treatment-resistant brain malignancies. Despite advances in surgery, radiation, and chemotherapy, prognosis remains poor due to the tumor’s heterogeneity, invasiveness, and immunosuppressive microenvironment. Traditionally, glioma pathogenesis has been attributed to genetic, epigenetic, and environmental factors. However, growing evidence suggests a significant role for the gut microbiota in central nervous system (CNS) regulation—including neuroinflammation, blood-brain barrier (BBB) integrity, and immune surveillance—raising the possibility that microbial communities may influence brain tumor progression and therapeutic response.
The gut-brain axis is now recognized as a critical immunological interface, where the microbiota can modulate systemic and CNS-specific immune responses. In glioma, dysbiosis may contribute to chronic inflammation, immune evasion, and resistance to immune checkpoint inhibitors (ICIs). At the same time, specific microbial taxa may enhance antitumor immunity and improve immunotherapy outcomes. These dualistic roles underscore the complexity of microbiota-cancer interactions.
The microbiota also impacts the pharmacokinetics, metabolism, and efficacy of brain tumor treatments. For example, microbial enzymes can alter drug bioavailability or promote resistance through epigenetic remodeling. Meanwhile, tumor heterogeneity further complicates therapy by fostering clonal subpopulations with variable sensitivities. Mechanisms such as PD-L1 overexpression and recruitment of immunosuppressive cells [e.g., regulatory T cells (Tregs), MDSCs, tumor-associated macrophages (TAMs)] contribute to glioma’s ability to evade immune detection and resist treatment.
This review aims to provide an overview of the emerging role of gut and intratumoral microbiota in the pathogenesis and treatment of glioma, with particular emphasis on their influence on tumor immunity and response to immunotherapy. The multifaceted interactions between the gut and intratumoral microbiota and glioma biology is explored. We examine their roles in modulating immune responses, shaping the tumor microenvironment, and influencing the efficacy of current and emerging therapies, with a particular focus on microbiota-informed strategies to enhance immunotherapy in neuro-oncology. We also discuss current microbiota-based strategies and future research directions.
Recent research has highlighted the intricate relationship between the gut microbiota and CNS function, forming the basis of the gut-brain axis. Through immune, metabolic, and neuroendocrine signaling pathways, gut microbes influence systemic inflammation, BBB integrity, and neuroimmune homeostasis. These interactions have important implications in brain tumor pathogenesis and therapy, positioning the microbiota as both a modulator and potential therapeutic target in glioma [1].
Brain cancer develops through a multistep accumulation of genetic and epigenetic mutations [2]. While only a small percentage of brain tumors are attributable to inherited mutations [3], the acquisition of such alterations enables malignant cells to evade regulatory mechanisms such as apoptosis and immune surveillance [4]. Tumor heterogeneity—both intra- and inter-patient—poses a significant challenge to the development of universal therapeutic strategies. Furthermore, during gliomagenesis, a pronounced state of immunosuppression is established, creating an environment conducive to tumor progression [5]. Tumor cells actively promote this suppression through the expression of immune checkpoint molecules such as CTLA-4 and PD-1, thereby disrupting antitumor immunity [6]. Immunotherapies are designed to counteract these mechanisms and reinvigorate the immune system against tumor cells [7]. In GB, immunosuppression is a hallmark, with elevated numbers of Tregs contributing to poor immune responses [8, 9]. The abundance of Tregs correlates with glioma grade, and their depletion has shown survival benefits in murine models [10, 11]. Current immunotherapeutic strategies, including monoclonal antibodies targeting inhibitory pathways, are under investigation and often combined with radiotherapy to enhance tumor immunogenicity [12, 13].
The gut microbiota may play a multifaceted role in glioma development and therapeutic response (Table 1). Bacteria and gut microbiota play a role in the biological processes of the tumor and specific immune recognition of tumor antigens [14]. In glioma patients, a significant reduction in gut microbial diversity has been shown compared to healthy controls. An increase in the family Bacteroidaceae and family Peptococcaceae was correlated with high risk, whereas family Ruminococcaceae was correlated with a protective effect against GB [15, 16]. It has been found that Bifidobaterium positively influence the number of activated antgen-presenting cells and Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium are associated with clinical response to anti-PD-1-based immunotherapy [17, 18]. The GB microenvironment presents major challenges in achieving a durable and effective anti-tumor response [19]. Gut microbiota-derived metabolites may impact the BBB permeability [20]. Bacterial strains isolated from the feces of healthy human donors are capable of strongly inducing interferon-γ-producing CD8⁺ T cells within the intestinal mucosa [21] on the other hand it is also important to consider the antibacterial impact of many drugs [22]. Folic acid produced by Bifidobacterium, which is associated with MGMT DNA methylation, may influence the therapeutic response to temozolomide (TMZ) [23]. In recurrent malignant gliomas patients, the composition of the gut microbiome showed higher relative abundance of Firmicutes, Bacteroidetes, Actinobacteria and lower relative abundance of Bacteroidetes and Cyanobacteria in bevacizumab and TMZ combination treatment compared with TMZ monotherapy [24].
Key microbiota-related factors influencing brain tumor progression and therapy response
| Factor | Mechanism | Impact on brain cancer |
|---|---|---|
| Dysbiosis | Inflammatory cytokine release | Promotes tumor progression |
| Probiotics | Modulation of immune cells | Enhances ICI efficacy |
| Antibiotics | Microbiota depletion | Reduced therapy response |
| Bacterial tropism | Targeted delivery | Increases drug specificity |
| Microbiota metabolites | Epigenetic regulation | Alters tumor gene expression |
| Intratumoral bacteria | Local immune effects | Modulates microenvironment |
ICI: immune checkpoint inhibitor
It is particularly intriguing that males are more prone to brain cancer, especially considering the critical role of bacterial colonization in shaping the postnatal development of the immune and endocrine systems key regulators of CNS function [25].
Fusobacterium, Longibaculum, Intestinimonas, Pasteurella, Limosilactobacillus, and Arthrobacter were found to be significantly enriched in animal model glioma tissues compared to adjacent normal brain tissues [26]. Microbiota-derived metabolites like short-chain fatty acids (SCFAs) promote Treg differentiation and immune tolerance [27]. Bacterial outer membrane suppresses tumor by interferon-γ-mediated antitumor response [28]. Metabolites such as imidazole lactate, N4-acetylcytidine, 1-ribosyl-imidazoleacetate, 1-stearoyl-2-oleoyl-GPE, 1-palmitoyl-2-linoleoyl-GPE, androstenediol monosulfate, 1-stearoyl-2-linoleoyl-GPE, 1-stearoyl-2-arachidonoyl-GPE, 1-palmitoyl-2-arachidonoyl-GPE, 1-oleoyl-2-arachidonoyl-GPE, 1-oleoyl-2-linoleoyl-GPE, Pimeloy lcarnitine/3-methyladipoyl carnitine, dihomo-linoleoylcarnitine, 1-palmitoyl-2-oleoyl-GPE, X-15523 were found to be associated with an increased risk of GB [29]. Tryptophan metabolites promote tumor cell proliferation in gliomas [30]. Acetate and glucose influence the modulation of isocitrate dehydrogenase (IDH), an enzyme whose mutation represents a significant molecular hallmark of glioma [31].
Following antibiotics induced dysbiosis, glycine increases angiogenesis, glioma growth and progression [32]. Glioma tumor cells’ aggressive growth depends on abundance of amino acids such as asparagine [33]. Zonulin, a protein influencing tight junctions, in both the tumor and serum of glioblastoma patients is higher in patients with poor prognosis [34].
Ongoing clinical trials, such as the phase 1/2 study assessing pembrolizumab (an anti-PD-1 antibody) in combination with radiotherapy in patients with recurrent glioblastoma (Clinical Trial ID: NCT04977375), and a trial investigating the TROP2-targeting antibody-drug conjugate LCB84 as a monotherapy or in combination with an anti-PD-1 antibody in advanced solid tumors, including glioblastoma, are exploring the therapeutic potential of PD-1 blockade in combination with other modalities. Given the emerging role of the gut microbiome in modulating immunotherapy responses, it will be critical to investigate its potential influence on outcomes in these and other approaches using PD-1 blockers. Sonication may improve the delivery of checkpoint inhibitors such as PD-1 blockers in GB [35]. Specific commensals regulate the response to immune therapy by modulating inflammation and adaptive immunity [36]. Bifidobacterium pseudolongum, enhances efficacy of ICIs through the production of the metabolite inosine [37]. The effectiveness of CTLA-4 blockade has been associated with the presence B. fragilis and/or B. thetaiotaomicron and Burkholderiales and interleukin 12 (IL-12)-dependent Th1 immune response [38]. Akkermansia muciniphila, which is enriched in patients that respond to treatment, promotes the secretion of the pro-inflammatory cytokine IL-12 by dendritic cells [39].
Antibiotic use correlates with poor PD-1 blockade outcomes in epithelial cancers and diminished levels of A. muciniphila [40]. Notably, supplementation with A. muciniphila can restore responsiveness to PD-1 blockade in fecal transplant models [40]. Additionally, gut microbiota composition affects the pharmacokinetics and efficacy of GB therapies such as UniPR1331, an Eph-ephrin antagonist [41]. The abundance of Bacteroidia and downregulation of Foxp3 is associated with accelerated glioma progression [42].
Therapies such as bevacizumab (antibody against VEGF), lomustine (chemotherapy), and ICIs have not shown significant survival benefits in GB [43–45]. Additional biomarkers such as high tumor mutation burden may improve the efficacy of the treatments [46]. The ketogenic diet may represent a promising therapeutic strategy for glioma by simultaneously targeting tumor metabolism and modulating the gut microbiome, through its effects on glucose and insulin signaling, neurotransmission, oxidative stress, inflammation, and gene expression via epigenetic mechanisms [47].
The mesenchymal subtype of glioblastoma, which has significant high expression of IL-6 and IL-6R, has been found associated with more aggressive, invasive, angiogenic, hypoxic, necrotic, inflammatory, and multitherapy-resistant [48]. IL-6 is a cytokine that induces neurogenesis and B-cell differentiation [49]. IL-6 suppresses the effectiveness of immune-checkpoint inhibition with anti-PD-L1 blockade [50]. Inhibition of IL-6 signaling in orthotopic murine glioma models reduces myeloid PD-L1 expression, diminishes tumor growth, and increases survival [51]. Probiotics have been shown to reduce IL-6 levels and may offer therapeutic benefits in the context of glioma [52, 53].
SCFA-induced activation of the NLRP3 inflammasome in the gut epithelium is another mechanism by which microbiota influence CNS inflammation. This process is relevant to both glioma progression and neuropsychiatric disorders characterized by elevated proinflammatory cytokines [54]. Moreover, aging-associated changes in microbiota heighten systemic inflammation, exacerbating outcomes in conditions such as stroke and possibly GB [55]. The influence of microbiota on adoptive cell-transfer therapies—by modulating Th2 and cytotoxic T cell responses—is an emerging area of research [56, 57].
ICIs, such as CTLA-4 and PD-1/PD-L1 antibodies, have transformed treatment paradigms for various cancers by reactivating anti-tumor T-cell responses. However, their application in neuro-oncology remains challenging due to the CNS’s relative immune privilege and the immunosuppressive glioma microenvironment. Some clinical trials have shown modest benefits in glioma patients, but overall response rates remain low. In addition, ICIs are associated with immune-related adverse events (irAEs), including encephalopathy, meningitis, myelitis, and cerebellar ataxia necessitating improved patient stratification and toxicity management [58]. ICIs efficacy remains limited in “cold” tumors such as brain tumors; however, growing evidence linking DNA damage repair mechanisms to immune responsiveness offers a potential breakthrough for expanding immunotherapy in this context [59]. In addition, it has been demonstrated that Siglec-9 functions as an immune checkpoint molecule on macrophages and can be targeted to enhance the therapeutic efficacy of anti-PD-1/PD-L1 treatments in glioblastoma mouse model [60]. Given the limited efficacy of combining IL-12 gene therapy with PD-1/PD-L1 inhibitors in glioblastoma, silencing the immunosuppressive long noncoding RNA INCR1 has emerged as a promising strategy to enhance IL-12-mediated antitumor immunity and potentially improve immunotherapy outcomes [61, 62]. Circadian rhythms significantly influence immune function, and incorporating chronotherapy and personalized treatment schedules has been proposed to optimize immunotherapy precision in brain cancers [63]. In tumor models, the angiotensin receptor blocker losartan significantly reduced anti PD-1 induced edema and “hot” tumor immune signature has been identified, providing both rationale and biomarkers for clinical evaluation of this combination in glioblastoma patients [64].
Emerging evidence indicates a bidirectional interaction between GB and the gut microbiota, in which both the tumor and its treatment modalities—particularly radiotherapy and chemotherapy—can profoundly affect microbial composition and function. These treatments, while essential for tumor control, can significantly reduce microbial diversity, disrupt the balance between commensal and pathogenic bacteria, and compromise intestinal barrier integrity. Glioma patients and preclinical models such as the GL261 orthotopic glioma model revealed significant alterations in gut microbiota composition during glioma progression and treatment [65]. Gut microbial profiles and associated metabolites correlated with TMZ pharmacodynamics, while antibiotic-induced microbiota depletion accelerated tumor growth, reduced TMZ efficacy, and impaired immune cell (macrophage and CD8α⁺ T cell) recruitment [66]. Glioma progression significantly altered microbial composition, and TMZ treatment was associated with increased levels of Akkermansia and Bifidobacterium, as well as preservation of Anaerotruncus, suggesting these microbial shifts may contribute to TMZ’s anti-tumor effects [67]. Using a humanized microbiome (HuM) model in a preclinical glioblastoma mouse model, researchers found that certain microbial communities such as Bacteroides cellulosilyticus may promote responsiveness to anti-PD-1 immunotherapy [68]. In advanced glioma, altered levels of SCFAs and microbial tryptophan catabolites that bind aryl hydrocarbon receptor [69] may reflect altered gut microbiota such as Bacteroides, Lactobacillus, Bifidobacterium, and Clostridium. Dysbiosis-related reductions in beneficial commensals, such as Bifidobacterium and Lactobacillus, further impair immune surveillance and can influence the host’s response to ICIs and chemotherapy. These findings suggest that therapeutic modulation of the microbiota may help restore microbial equilibrium and enhance therapeutic efficacy in glioma patients.
Microbial metabolites such as SCFAs, bile acids, and tryptophan catabolites have emerged as critical mediators in the crosstalk between the microbiota and tumor microenvironment. These metabolites can cross the BBB and influence glioma progression by modulating immune responses and altering gene expression in both tumor and immune cells. SCFAs like butyrate exert broad health-promoting and anti-tumor effects by modulating immune responses, shaping the tumor inflammatory microenvironment, inhibiting tumor cell proliferation, enhancing immunotherapy efficacy, and preserving intestinal epithelial barrier integrity [70]. Acetate can serve as a nutritional source for cancer cells through its conversion to acetyl-CoA [71], a process implicated in glioblastoma growth. Microbiota-derived metabolites have been implicated in the regulation of neutrophil chemotaxis, induction of Treg development, IL-10 secretion, inhibition of NF-κB and the suppression of cytokine production from myeloid cells [72]. Furthermore, tryptophan, the sole precursor of serotonin, produced by the microbiota may also contribute to the reprograming of CD4⁺ T cells into immunoregulatory intraepithelial T cells [73]. Microbial products also influence the expression of non-coding RNAs, including microRNAs and long non-coding RNAs (lncRNAs), which may regulate critical signaling pathways such as PI3K/AKT and STAT3 involved in glioma proliferation and resistance [74, 75]. Understanding the role of these microbial-derived molecules offers novel opportunities for therapeutic targeting and biomarker development in glioma.
The recent discovery of bacterial DNA and viable microbes within CNS malignancies has fundamentally challenged the longstanding notion of the brain as a sterile and immune-privileged site. Increasing evidence suggests that the glioma microenvironment may harbor a diverse repertoire of microbial populations, such as Acinetobacter, Neisseria macacae and Enterobacter cloacae in glioblastoma with the potential to influence tumor development, immune dynamics, and therapeutic responsiveness [76, 77]. These findings open new avenues in neuro-oncology, emphasizing the need to understand how bacteria enter the CNS and what functional roles they may play once established within the tumor milieu.
Several plausible mechanisms have been proposed to account for microbial colonization in glioma. One prominent pathway involves immune cell-mediated trafficking, often described as the “Trojan horse” mechanism. In this scenario, peripheral immune cells such as monocytes and dendritic cells engulf microbial cargo in the gut or systemic circulation and migrate to the brain as part of immune surveillance or during inflammatory states. These immune cells may subsequently release intracellular bacteria into the brain parenchyma, facilitating microbial seeding within gliomas [78]. Another key route is the disruption of the BBB, a hallmark of high-grade gliomas such as GB [79]. This disruption may permit the translocation of circulating bacteria and microbial-associated molecular patterns (MAMPs), such as lipopolysaccharides and bacterial nucleic acids, into the tumor microenvironment. Moreover, other speculative yet biologically plausible conduits include retrograde axonal transport along cranial nerves and entry via the recently characterized meningeal lymphatic system—routes increasingly recognized as potential highways for immune cell and microbial traffic [80]. Moreover, a shared lymphatic circuit between the posterior eye and the brain enables a coordinated immune response, revealing an underexplored immunological connection and offering potential for novel therapeutic approaches in CNS diseases [81].
Once established within the CNS, intratumoral microbes may actively shape the immune and metabolic landscapes of gliomas. In oral squamous cell carcinoma and colorectal cancer, the distribution of the microbiota within a tumor is organized in microniches with immune and epithelial cell functions that promote cancer progression [82]. Bacterial components such as flagellin, lipoteichoic acids, and peptidoglycan can engage pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), expressed on both resident microglia and infiltrating immune cells [83–87]. This interaction can initiate a cascade of immune signaling, resulting in either proinflammatory responses that may support antitumor immunity or immunosuppressive pathways that foster tumor growth and immune evasion. In addition to immune modulation, microbial metabolites and structural elements can induce epigenetic changes within tumor cells. SCFAs such as butyrate and propionate, commonly produced by anaerobic bacteria, can influence histone acetylation and DNA methylation patterns, thereby altering gene expression programs [88]. Other bacterial molecules, including nucleic acids and cell wall fragments, can modulate non-coding RNA profiles and chromatin remodeling enzymes, potentially enhancing tumor cell plasticity and contributing to therapeutic resistance [89]. Certain bacteria may also metabolize chemotherapeutic agents into inactive forms, potentially reducing treatment efficacy and contributing to drug resistance [90]. These findings suggest that microbes are not merely passive bystanders but active agents capable of rewiring tumor biology at the epigenetic level.
While preclinical studies and early clinical observations support the therapeutic promise of microbiota-targeted interventions such as probiotics, postbiotics, and fecal microbiota transplantation (FMT), their clinical application in neuro-oncology remains limited. The prospect of modulating the tumor microbiome to enhance therapy or mitigate resistance is an exciting and rapidly evolving frontier.
Despite the enthusiasm surrounding the role of microbiota in glioma, significant uncertainties and methodological challenges remain. A central controversy involves the very existence of intratumoral microbiota within the CNS. Although several high-throughput sequencing studies have identified bacterial DNA and RNA in glioma specimens [75], others have raised concerns regarding potential contamination, batch effects, and the misclassification of sequencing artifacts as true microbial signals [88, 89]. The low microbial biomass typical of CNS tissues makes them particularly vulnerable to contamination during sample collection and processing, thereby complicating data interpretation.
Adding to this complexity is the substantial interindividual variability observed in both gut and intratumoral microbiota. Factors such as geography, dietary habits, medication use, and sample processing techniques all contribute to this heterogeneity, making cross-study comparisons difficult and hindering the identification of reproducible microbial signatures [90]. As a result, current findings often lack external validation, and their clinical utility remains uncertain.
Perhaps the most pressing knowledge gap is the distinction between correlation and causation. While many studies report associations between specific microbial taxa or metabolites and tumor characteristics or immune phenotypes, robust mechanistic evidence of causality is often lacking. This is particularly problematic in human studies, where confounding factors—such as prior antibiotic exposure or systemic immunosuppression are difficult to control. The use of germ-free (GF) or gnotobiotic mouse models and precision microbial perturbation techniques could offer more definitive insights, yet these approaches remain underutilized in glioma research.
Finally, the clinical translation of microbiota-based therapies faces several unresolved questions. The safety and efficacy of interventions such as FMT, engineered probiotics, or bacterial lysates in neuro-oncology patients remain largely theoretical. Given the immunocompromised status of many glioma patients, rigorous clinical trials with clearly defined endpoints and standardized protocols are essential to assess the risks and benefits of such approaches. Only through carefully designed translational studies can the full therapeutic potential of the microbiome in glioma be realized.
Emerging evidence highlights the complex and bidirectional role of the microbiota in glioma progression and treatment response. Microbial taxa and their metabolites influence tumor biology through diverse mechanisms, including epigenetic reprogramming, immune suppression, and chronic inflammation, thereby contributing to tumor growth and recurrence (Figure 1). While certain bacteria and their metabolic products foster immunosuppressive conditions, others enhance antitumor immunity and may augment the efficacy of ICIs. This functional duality presents both a therapeutic challenge and an opportunity: with precise modulation, microbiota-targeted approaches could shift the immune milieu toward tumor suppression while minimizing oncogenic risks.
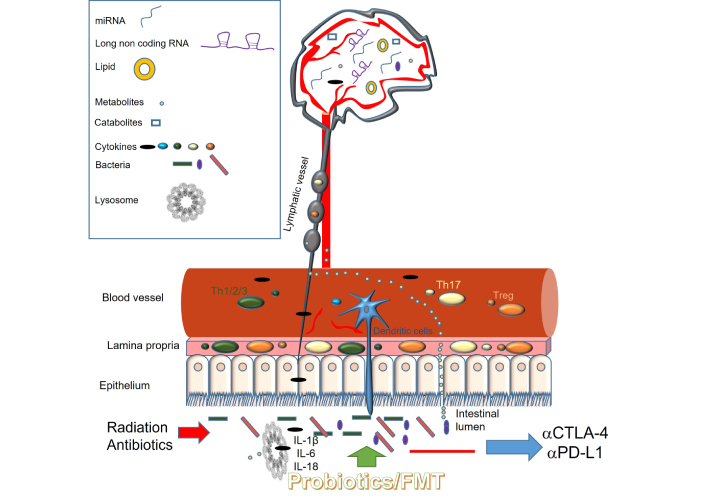
Proposed mechanisms by which the gut microbiota influences the brain and brain tumors. Treg: regulatory T cell; IL: interleukin
Despite promising preclinical findings, translating microbiome-based insights into clinical applications in neuro-oncology remains challenging. Murine models, while informative, do not fully replicate the immunological and microbial complexity of human gliomas. Furthermore, interspecies differences in microbiota composition and metabolite profiles hinder direct extrapolation to patients. Clinical trials evaluating microbiota modulation via probiotics, dietary interventions, or FMT—as adjuncts to immunotherapy are underway, but robust data on their safety, efficacy, and long-term impact in glioma are still limited.
One of the most pressing needs is the identification of reliable microbiota-derived biomarkers to guide immunotherapy use. These may include specific taxa, metabolites, or host immune signatures influenced by the microbiome. However, clinical implementation is hampered by technical variability and the absence of standardized protocols for sample collection, sequencing, and data analysis. Harmonization across platforms and integration of multi-omic tools—such as metagenomics, metabolomics, transcriptomics, and immune profiling—are essential to develop reproducible and clinically meaningful biomarkers.
The limited responsiveness of gliomas to ICIs is partially attributed to their highly immunosuppressive tumor microenvironment. Microbiota-based strategies including probiotic supplementation, FMT, and engineered microbial vectors represent promising avenues to sensitize tumors to immunotherapy. However, the risk of inducing or exacerbating irAEs, particularly in immunocompromised patients, remains underexplored and warrants thorough investigation.
Looking ahead, several research priorities must be addressed. Mechanistic studies using clinically relevant glioma models should clarify how individual microbes and their metabolites influence immune dynamics, epigenetic states, and treatment resistance. Longitudinal studies combining deep microbial and immune profiling could uncover temporal changes that correlate with therapy response or resistance. Incorporating microbiome-informed patient stratification into clinical trial design may also enhance therapeutic outcomes.
Recent advances in microbial engineering and personalized medicine offer new translational possibilities. Engineered tumor-homing bacteria can deliver therapeutic agents directly into the tumor niche, bypassing the BBB and modifying the immune environment in situ. Personalized microbiota interventions, such as tailored probiotics or autologous FMT—may restore beneficial microbial communities and metabolic outputs that support antitumor immunity. Additionally, microbiota-derived biomarkers could inform patient selection for immunotherapy, enabling a precision oncology approach in glioma care.
Recent exploratory work has begun to shed light also on fungal communities (mycobiota) within brain tumors and their surrounding environments [91]. In a preliminary study involving five randomly selected brain tumor patients, researchers examined the microbiota and mycobiota profiles of tumor tissue, tumor-adjacent tissue, blood, and gut samples [92]. While some correlation was observed between bacterial communities across these compartments, the fungal composition within the tumors exhibited a unique and independent pattern. Notably, the tumor-associated mycobiota differed significantly from fungal profiles in the gut, blood, and surrounding tissues, suggesting that fungi may play a distinct role in the tumor microenvironment. Furthermore, the effects of the mycobiome were not redundant with those of the bacterial microbiome, indicating separate immunological or metabolic functions. These findings underscore the need for further investigation into the specific contributions of fungi in brain tumor biology and raise the possibility that the tumor-associated mycobiota may represent additional therapeutic or diagnostic targets, particularly if specific fungal taxa correlate with tumor subtypes or disease progression.
The gut microbiota can modulate brain function and tumor progression via multiple interconnected pathways. Microbial components and metabolites in the intestinal lumen interact with the host immune system, influencing the activity of immune cells, cytokine release, and the production of bioactive molecules such as SCFAs, lncRNAs, and microRNAs. These mediators can affect the CNS either directly, by crossing the BBB, or indirectly, through systemic immune signaling. Activation of inflammasome pathways by microbial signals leads to increased production of proinflammatory cytokines, including IL-1β, IL-6, and IL-18, contributing to a tumor-promoting microenvironment. External interventions such as radiation therapy and antibiotic use can disrupt gut microbial homeostasis, with downstream effects on immune surveillance and the efficacy of immunotherapy. Targeted strategies to restore microbial balance may enhance anti-tumor immunity and therapeutic outcomes.
Ultimately, integrating microbiome science into neuro-oncology may help overcome the immunological barriers and therapeutic resistance that define gliomas. Personalized, microbiota-informed strategies have the potential to improve immunotherapy responses, reduce adverse effects, and offer more effective, individualized treatment pathways for patients with this devastating disease.
BBB: blood-brain barrier
CNS: central nervous system
FMT: fecal microbiota transplantationf
GB: glioblastoma
GF: germ-free
ICIs: immune checkpoint inhibitors
IL-12: interleukin 12
SCFAs: short-chain fatty acids
TLR: Toll-like receptor
TMZ: temozolomide
Tregs: regulatory T cells
RMS: Conceptualization, Writing—original draft, Writing—review & editing.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1862
Download: 23
Times Cited: 0
Haim Werner, Ilan Bruchim
