Affiliation:
1Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), 119435 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0003-1592-5703
Affiliation:
1Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), 119435 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0003-0485-6802
Affiliation:
1Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), 119435 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0001-8335-1380
Affiliation:
2Kazan Federal University, 420008 Kazan, Russian Federation
Email: mtrushin@mail.ru
ORCID: https://orcid.org/0000-0001-7467-011X
Explor Med. 2025;6:1001336 DOl: https://doi.org/10.37349/emed.2025.1001336
Received: January 22, 2025 Accepted: May 06, 2025 Published: June 23, 2025
Academic Editor: Maria Paz Ocaranza, Pontificia Universidad Católica de Chile, Chile
Aim: To assess the effect of a lipophilic angiotensin-converting enzyme inhibitor (ACEI) in combination with a calcium antagonist (CA) on the 24-hour blood pressure (BP) profile, systemic inflammation in patients with arterial hypertension (AH) and metabolic disorders (MD).
Methods: Fifty-eight patients with the ≥ 2nd degree of AH was divided into 3 groups: patients with AH without metabolic syndrome (MS), patients with AH and MS, and patients with AH and diabetes mellitus (DM). Taking into account the BP profile characteristics, therapy with ACEI perindopril and CA amlodipine in a fixed combination (FC) was prescribed. The observation period for patients was 12 weeks.
Results: A profile with an insufficient decrease in BP at night was more often detected in persons with MS having DM and nocturnal hypertension. In patients with AH and DM, the values of daily BP variability exceeded those in persons without MS (P < 0.05). Patients with MS had a higher concentration of high-sensitivity C-reactive protein (hsCRP) compared to patients without MS (3.5 mg/L; P < 0.01). Patients with DM and AH achieved target BP in 60% of cases during treatment: office BP decreased to 134.8 (17.97 kPa) ± 8.7/83.2 (11.09 kPa) ± 6.7 mmHg (∆ = –31/–16 mmHg), 90% of patients required maximum therapeutic doses of antihypertensive therapy (AHT). A decrease in the hsCRP concentration was detected (P < 0.05) in patients of groups 2 and 3, which showed practical possibility of average/maximum therapeutic doses influence on the activity of systemic inflammation (∆ = –12.8% in patients group 2 and ∆ = –11.2% in patients of group 3).
Conclusions: A combination of a lipophilic ACEI and a vasoselective СA promotes good BP control, a decrease in the activity of systemic inflammation, and hypersympathicotonia in patients with MD.
Despite the recent progress achieved in domestic and world medicine, the arterial hypertension (AH) incidence remains high and amounts to 30–45% in Russia (2024); according to the epidemiological study ESSE-RF-2 (epidemiology of cardiovascular diseases and the risk of their impact in the regions of the Russian Federation-2; 2019)—44.2% [1]. Despite relatively good awareness of their AH (76.8% of women and 69.4% of men), less than 25.0% of them control BP. Failure to achieve target BP is due to many reasons: long-term and/or combined influence of risk factors (RFs), high patient comorbidity, persistent MD [1, 2].
Achieving target BP in certain clinical situations has prognostic significance. In these situations, patients with AH and diabetes mellitus (DM), and abdominal obesity/metabolic syndrome (MS) deserve special attention. This category of patients has the highest risk of developing cardiovascular complications, so achieving the target BP level quickly is the main strategic goal [3]. For this purpose, experts recommend the use of two and/or more drugs in this category of patients already at the start of antihypertensive therapy (AHT) [4]. The most justified step is to prescribe a calcium antagonist (CA) of the dihydropyridine series and an angiotensin-converting enzyme inhibitor (ACEI) in a fixed combination (FC) [3, 5]. In addition to a pronounced antihypertensive effect, the CA ability to improve endothelial function, exhibit antiatherogenic properties, reduce the albuminuria severity, and slow down the nephrosclerosis progression determined a special indication for prescribing this class—MD and diabetic nephropathy [6]. The ACEI’s advantages are associated with their ability to suppress the activity of neurohumoral systems that play a fundamental role in the hypertension pathogenesis—sympathoadrenal system (SAS) and renin-angiotensin-aldosterone system (RAAS) [7]. The ACEI’s ability to influence the processes of reverse pathological remodeling, have a pronounced organ protective effect in various clinical situations [3, 8–10], improve the disease prognosis has been confirmed by large-scale studies EUROPA [11], PREAMI [12], PROGRESS [13], HOPE [14], SECURE [15], ASCOT [16], etc., which allows this class of antihypertensive medications to occupy the AHT leading position for many years. In addition, ACEIs as part of FC significantly improve the dihydropyridine CA tolerability profile due to their antisympathetic effects.
The aim of this study was to determine the effect of the lipophilic ACEI perindopril in combination with the slow calcium channel blocker of dihydropyridine group amlodipine on the 24-hour blood pressure (BP) profile and systemic inflammation in patients with AH and MD.
The present study included 58 outpatients of the FSAEI HE I.M. Sechenov First MSMU of MOH of Russia (Sechenovskiy University) with the 2nd (67.2%) and 3rd degree (32.8%) AH according to systolic BP (SBP) and/or diastolic BP (DBP) (35 women and 23 men; age 60.9 ± 12.8 years). The majority of participants had an insufficient response to previously prescribed therapy (62.1%) and were included in the study after a washout period. Exclusion criteria were stage 1 hypertension; symptomatic hypertension; chronic heart failure Classes III and IV (the NYHA); instability of coronary and/or cerebral blood flow less than 6 months ago; DM decompensation; pregnancy and lactation of women; sensitivity to any of the drugs studied. AH was diagnosed based on Russian recommendations (2024). The AH degree was determined according to average values of three-time office BP measurements [3].
The obesity degree was determined according to the World Health Organisation criteria [with a body mass index (BMI) of more than 30 kg/m2]. MS was diagnosed taking into account the Recommendations of the Russian Society of Cardiologists for MS diagnosis and treatment (2009): abdominal obesity (waist circumference > 80 cm in women and > 94 cm in men) in combination with ≥ 2 additional criteria: AH (BP ≥ 140/90 mmHg); triglycerides (TG) more than 1.7 mmol/L; high-density lipoprotein (HDL) cholesterol (CH) less than 1.2 mmol/L in women and less than 1.0 mmol/L in men; impaired glucose tolerance (IGT), impaired fasting glucose (IFG), or a combination of both [17]. According to the study design, 4 visits were provided: B1-inclusion visit, B2-3-control visits after 2, 4 weeks, B4-final visit after 12 weeks. During B1 and B4, in addition to a physical examination with anthropometric parameters assessment, the following laboratory tests were performed: high-sensitivity C-reactive protein (hsCRP) with a lower detection limit of 0.1 mg/L, uric acid (UA), creatinine, basal glucose and glycated hemoglobin (HbA1c if there is DM), if prescribed, 2 hours after a carbohydrate load (for the purpose of diagnosing MS), HbA1c (if there is DM), total CH and TG. By calculation, a laboratory assessment of the CH level not associated with HDLs (non-HDL-C) was carried out using the formula non-HDL-C=C-HDL-C, as well as LDL-C as the difference in non-HDL-C-(TG/3–0.14) [18]. The instrumental examination included electrocardiography in 12 standard leads using the Schiller Cardiovit AT-1 device [to assess heart rhythm, heart rate (HR) and detect arrhythmias] and automatic non-invasive BP registration and pulse rate with a 24-hour ambulatory BP monitoring (ABPM) (device “BPLab”, LLC “iPetr Telegin”). The averaged values for all time periods of SBP and DBP, pulse BP (PBP), time index (TI), area index (AI), general hyperbaric index (GHI), SBP and DBP were determined. The sympathetic nervous system (SNS) activity was assessed by BP variability (BPV) and quantitative analysis of HR RR intervals. BPV was defined as the standard deviation of the mean value over the day separately for systolic (Daily SBP BPV) and DBP (Daily DBP BPV).
After being examined, participants were divided into 3 groups. Observation group 1 (n = 23) included patients with AH without MS (with a normal BMI without lipid and carbohydrate metabolism disorders), group 2 (n = 25) included patients with AH and MS (with abdominal obesity and various MD), group 3 included patients with AH and DM (n = 10). Patients of groups 1 and 2 comparison groups didn’t have DM. Initially in the group 1 SBP office (mmHg) was 162.7 ± 10, in the 2nd—163.2 ± 10.5, in the 3rd —166.2 ± 12.5.
Having analysed previous therapy, study participants, in addition to lifestyle modification, were offered two drugs FC with predominantly renal elimination- amlodipine and perindopril (manufactured in Russia) in the evening at a starting dose of 5 mg/4 mg, with a possible subsequent increase to 10 mg/8 mg. If the antihypertensive effect was insufficient at the start of therapy, short-acting ACEI forms, ß-blockers or CAs were recommended. The AHT effectiveness was reviewed during control visits B2-3. The main criterion for the AHT effectiveness was the achievement of the target BP level, which was detected by office BP measurement and ABPM. Additional criteria were: the hospitalisation frequency during the observation period due to uncontrolled AH, the safety FC profile and the side effects frequency. It should be noted that patients with DM continued to take glucose-lowering drugs previously selected by the endocrinologist/general practitioner. The observation period for patients was 12 weeks.
The study was carried out taking into account international rules and ethical standards developed on the basis of the World Association’s Declaration of Helsinki «Ethical Principles for Medical Research Involving Human Subjects» [19]. The study was approved by the Local Ethics Committee of Sechenov University (Extract from the Protocol of the 17–24 meeting dated 04.07.2024). The obtained data from the study participants was entered into tables in the Microsoft Office Excel application. Statistical processing of the results was carried out using STATISTICA 10.0, «StatSoft». Standard descriptive statistics methods were used for calculations. Quantitative data are presented as the median Me, 25 and 75 percentiles (LQ, UQ), qualitative features—as the absolute number of patients with a given feature and percentages of their number in the group. Given the small samples, comparisons of medians between groups were performed using the Mann-Whitney test. The significance of the differences obtained was taken to be P < 0.05.
The main demographic characteristics for the three patient groups are presented in Table 1.
General characteristics of the groups of patients
| Indicator | Group 1АН without MS(n = 23) | Group 2AН and MS(n = 25) | Group 3AН and DМ(n = 10) |
|---|---|---|---|
| Men/Women (n) | 11/12 | 11/14 | 1/9 |
| Age, years | 53.3 (40.9; 66.3) | 54.6 (44.7; 65.8) | 63.6 (49.3; 72.8)∆ |
| BMI, kg/m2 | 26.1 (20.3; 29.2) | 32.3 (30.7; 37.2)# | 33.6 (32.5; 37.6)∆ |
| SBP office, mmHg | 162.7 (148.0; 173.0) | 163.2 (146.0; 172.0) | 166.2 (145.0; 180.0) |
| DBP office, mmHg | 95.6 (87.0; 102.0) | 98.7 (86.0; 108.0) | 99.7 (90.0; 105.0) |
| HR, bpm | 72.6 ± 9.4 | 74.6 ± 7.3 | 77.0 ± 10.6 |
| Components of metabolic syndrome | |||
| Waist circumference (women), cmWaist circumference (men), cmAdditional, % (n) BP over 140/90 mmHg Glycemia (≥ 6.1 and < 7 mmol/L) Triglycerides (≥ 1.7 mmol/L) HDL (< 1.0 mmol/L) LDL (> 3.0 mmol/L) | 77.6 (74; 79)90.3 (82; 91)100.030.44.391.395.7 | 89.3 (82; 100)101.6 (88; 118)#100.064.024.080.0100.0 | 96.4 (86; 110)∆103.0 (93; 127)∆100.00.020.0100.0100.0 |
# P < 0.05 comparing groups 1 and 2; ∆ P < 0.05 comparing groups 1 and 3. Quantitative data are presented as the median Me, 25 and 75 percentiles (LQ, UQ), and in the form of M ± m, where M is the mean value, m is the standard deviation in the case of their normal distribution. AH: arterial hypertension; MS: metabolic syndrome; DM: diabetes mellitus; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; BP: blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein
The BP level at the time of screening indicated grade 2 AH predominance in all groups, and in the general population reached a value of 162.9 ± 13.7/98.9 ± 9.4 mmHg. The groups of patients with and without MS didn’t have statistically significant differences in age (54.6 ± 10.3 and 53.3 ± 12.2; years) and duration of hypertension (11.9 ± 6.7 and 11.3 ± 5.8 years). Patients with DM were older (mean age 63.6 ± 9.8 years; P < 0.05), with a longer duration of AH (12.6 ± 9.0 years) and a high incidence of various rhythm disturbances (P < 0.05).
Long-term disorders of carbohydrate and lipid metabolism contributed to the formation of the ambulatory DBP profile characteristics in patients with MS and DM. In this category of patients, at all-time intervals, a higher level of BP was detected in comparison with the average values of patients with isolated hypertension. In patients with DM, in comparison with patients of groups 1 and 2, a greater hyperbaric daily load was found in terms of TI and AI (Table 2). The most significant differences were observed in mean PBP, SBP and DBP during the night period (P < 0.05).
Achieving the target blood pressure in patients of three groups by the end of the follow-up period
| Indicator | SBP, mmHg | DBP, mmHg | HR, bpm | Amlodipine 10 mg/perindopril 8 mg; % (n) | BP, mmHg | ||
|---|---|---|---|---|---|---|---|
| < 135/80 | ≤ 130/70–80 | ||||||
| Group 1АН without MS | Initially | 162.7 ± 8.8 | 95.6 ± 6.4 | 76.6 ± 9.4 | 0.0% | - | - |
| Week 4; ∆ | 139.4 ± 9.2; 14.3% | 88.3 ± 7.8; 7.7% | 70.8 ± 7.3; 7.6% | 30.4% (7) | - | - | |
| Week 12; ∆ | 132.6 ± 7.4; 18.4% | 82.2 ± 7.3; 14.0% | 68.3 ± 7.1; 9.8% | 39.1% (9) | 91.3% | 73.9% | |
| Group 2AН and MS | Initially | 163.2 ± 10.5 | 98.7 ± 8.3 | 78.6 ± 5.3 | 0.0% | - | - |
| Week 4; ∆ | 141.4 ± 10.5; 13.4% | 88.4 ± 9.2; 11.4% | 72.4 ± 8.7; 7.9% | 44.0% (11) | - | - | |
| Week 12; ∆ | 134.5 ± 8.3; 17.6% | 81.9 ± 8.7; 15.8% | 70.5 ± 7.4; 10.4% | 68.0% (17) | 82.6% | 68.0% | |
| Group 3AН and DМ | Initially | 165.2 ± 12.5 | 99.7 ± 7.4 | 74.0 ± 6.6 | 0.0% | - | - |
| Week 4; ∆ | 151.9 ± 9.6; 8.1% | 87.6 ± 8.3; 12.1% | 69.8 ± 7.2; 5.7% | 90.0% (9) | - | - | |
| Week 12; ∆ | 134.8 ± 8.7; 18.4% | 83.2 ± 6.7; 16.6% | 66.3 ± 5.4; 10.4% | 90.0% (9) | 80.0% | 60.0% | |
SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; BP: blood pressure; AH: arterial hypertension; MS: metabolic syndrome; DM: diabetes mellitus
Individuals with MS initially had a predominant profile with an insufficient decrease in SBP at night, while individuals with DM had nocturnal AH. In patients with AH and DM, the daily BPV values initially exceeded the SBP/DBP variability in patients of groups 1 and 2 by 14.1%/17.1% (P < 0.05) and 7.6% (P = n/a)/11.1% (P = 0.04). Daily SBP variability in patients of group 3, directly dependent on the level of UA (r = 0.64; P = 0.02), glucose (r = 0.82; P = 0.01), hsCRP (r = 0.73; P = 0.02), emphasised the need for strict BP control and feasibility of medium or high AHT doses at the initiation stage. In patients with MS, the SBP/DBP variability at night correlated with hsCRP (r = 0.42/0.29; P < 0.05), UA (r = 0.24; P = 0.04/r = 0.20; P = 0.058), and 24-hour SBP correlated with glucose (r = 0.33; P = 0.01) and hsCRP (r = 0.31; P = 0.02), indicating the role of systemic inflammation and MD in the AH pathogenesis.
The use of amlodipine/perindopril at a middle dose of 5/4 mg contributed to a significant decrease in office BP in all groups (P < 0.0001). However, it was necessary for the majority of patients with DM (90.0%) to increase the dose of amlodipine to 10 mg and perindopril to 8 mg during the second visit (after 4 weeks of treatment). During the second visit, the prescription frequency of double AHT doses in groups 1 and 2 was 30.4% and 44.0% (Table 2).
By the end of the observation period, in individuals with and without MS, the decrease in SBP/DBP was comparable to the initial level (–28/–17 mmHg and –30/–13 mmHg), as were the final BP mean values (134.5 ± 8.3/81.9 ± 8.7 mmHg and 132.6 ± 10.5/82.2 ± 7.3 mmHg (Figure 1). In patients with DM, the average BP reached 134.8 ± 8.7/83.2 ± 6.7 mmHg (∆ = –31/–16 mmHg). However, they more often required prescribing maximum therapeutic AHT doses. Patients in groups 1 and 2 received maximum FC doses in 39.1% and 68.0% of cases. The proportion of patients in group 1 who achieved a target BP level of ≤ 130/70–80 mmHg was 73.9%, group 2 was 68.0%, and group 3 was 60% (Table 2).
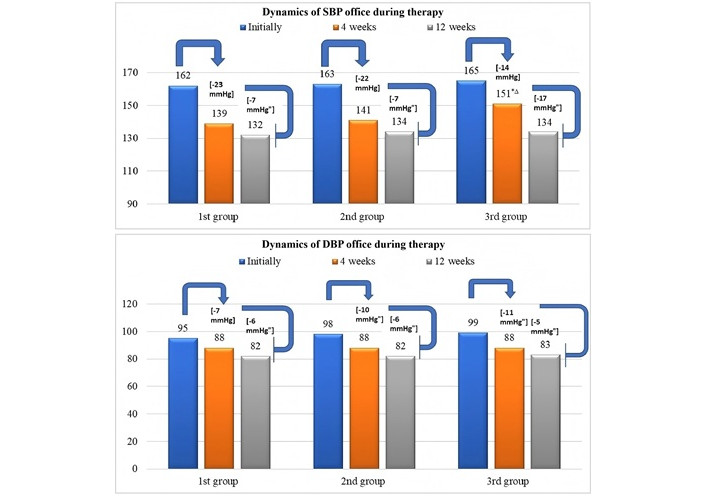
Dynamics of SBP and DBP levels after 1 and 3 months of observation. ∆ P < 0.05 comparing groups 1 and 3; * P < 0.05 comparing groups 2 and 3; " P < 0.05 for intragroup comparison (initial and at the end of the study). SBP: systolic blood pressure; DBP: diastolic blood pressure
Despite the comparable daily decrease in SBP/DBP in all groups, intergroup differences were established in the degree of decrease in indexed SBP indicators and total hyperbaric SBP load between patients of groups 1 and 3 (Table 3). SBP TI in patients of group 3 decreased by 58.4% (from 72.8 ± 8.5% to 30.3 ± 4.3%; P = 0.0001), SBP GHI by 66.0% (P = 0.0003), in patients of group 1 the above indicators dynamics were 48.2% and 58.8%, respectively (P < 0.05). Intergroup differences also manifested themselves in the degree of reduction in indexed DBP indicators, showing superiority in reducing diastolic load in the night and 24-hour period. Daily DBP TI in patients of group 1 decreased by 37.6% (from 45.2 ± 6.3% to 28.2 ± 5.7%; P = 0.0003), in representatives of group 3 by 49.7% (from 69.2 ± 7.4% to 35.1 ± 6.0%; P = 0.0001).
Comparative assessment of SMAD parameters in patients with hypertension on the background of ongoing therapy
| Indicator | Group 1of АН without MSM (95% CI) | Group 2AН and MSM (95% CI) | Group 3AН and DМM (95% CI) | |
|---|---|---|---|---|
| Daily SBP, mmHg | Initially | 163.1 (150.0–176.3) | 164.0 (149.5–173.4) | 167.8 (147.5–178.9)∆* |
| At the end of observation | 133.7 (110.0–149.4)" | 136.0(114.8–149.3)" | 138.2 (116.6–150.7)" | |
| Daily DBP, mmHg | Initially | 95.7 (89.1–98.3) | 96.2 (89.0–98.7) | 96.3 (87.4–99.3) |
| At the end of observation | 82.0 (64.6–86.8)" | 82.7 (70.7–93.8)" | 8.8 (69.8–89.0)" | |
| Day SBP, mmHg | Initially | 163.9 (150.9–192.6) | 165.3 (156.2–178.7) | 168.9 (148.3–179.5)∆* |
| At the end of observation | 137.2 (118.6–149.4)" | 138.2 (120.7–151.8)" | 138.9 (121.6–152.3)" | |
| Day DBP, mmHg | Initially | 96.8 (89.6–98.3) | 97.8 (90.0–98.7) | 98.0 (89.4–104.4) |
| At the end of observation | 83.0 (65.7–88.2)" | 83.4 (71.2–94.5)" | 83.9 (65.2–89.0)" | |
| Night SBP, mmHg | Initially | 140.2 (120.7–175.0) | 139.8 (108.8–159.2) | 142.8 (115.3–174.5) |
| At the end of observation | 127.8 (110.5–141.3)" | 130.3 (118.6–132.5)" | 131.8 (104.6–135.9)"∆ | |
| Night DBP, mmHg | Initially | 88.5 (76.0–96.5) | 91.2 (76.8–97.1)# | 93.1 (77.3–99.3)∆* |
| At the end of observation | 79.8 (72.6–85.9)" | 82.4 (73.7–90.2)" | 82.8 (65.6–84.7)"∆ | |
| Daily HR, bpm | Initially | 70.7 (60.3–90.4) | 73.3 (63.5–92.2) | 75.4 (62.0–93.5)∆ |
| At the end of observation | 69.6 (58.0–74.1)" | 70.7 (63.3–74.0)" | 70.3 (56.6–75.4)" | |
| Daily SBP TI, % | Initially | 65.6 (29.2–90.0) | 69.5 (30.8–97.5) | 72.8 (30.4–100.0)∆* |
| At the end of observation | 33.1 (12.2–48.5)" | 33.4 (16.1–54.2)" | 30.3 (13.7–54.2)"∆ | |
| Daily SBP AI, conventional units | Initially | 142.2 (122.2–231.0) | 140.5 (119.5–179.5) | 157.8 (123.0–304.4)∆* |
| At the end of observation | 43.3 (20.8–70.5)" | 67.9 (66.9–89.7)"# | 56.9 (43.8–101.2)"∆* | |
| Daily DBP TI, % | Initially | 45.2 (16.3–67.3) | 50.8 (26.7–72.3)# | 69.2 (20.4–82.4)∆* |
| At the end of observation | 28.2 (0.0–31.7)" | 22.4 (1.4–34.5)" | 35.1 (12.8–41.8)"∆ | |
| Daily DBP AI, conventional units | Initially | 89.4 (65.6–110.6) | 100.7 (85.2–133.9)# | 110.4 (72.3–150.6)∆* |
| At the end of observation | 33.9 (8.8–52.7)" | 40.6 (2.9–77.7)"# | 38.7 (4.8–85.3)"* | |
| Daily SBP GHI, % | Initially | 151.6 (128.6–189.6) | 163.2 (126.7–213.0) | 187.3 (129.0–220.2)∆* |
| At the end of observation | 62.4 (43.5–110.3)" | 72.3 (36.4–109.2)"# | 63.7 (24.8–99.7)"* | |
| Daily DBP GHI, % | Initially | 107.3 (86.4–142.8) | 107.1 (90.5–148.6) | 121.2 (87.2–161.1)∆* |
| At the end of observation | 48.3 (12.6–86.3)" | 50.7 (12.8–90.2)" | 53.8 (12.0–95.4)"∆* | |
| Daily SBP BPV, mmHg | Initially | 13.4 (6.7–18.3) | 14.5 (11.5–19.8)# | 15.6 (12.4–22.2)∆ |
| At the end of observation | 12.0 (7.0–14.5)" | 13.1 (6.6–16.4)" | 13.9 (8.9–19.0)"∆ | |
| Daily DBP BPV, mmHg | Initially | 9.7 (6.2–11.5) | 10.4 (6.3–11.6) | 11.7 (9.2–17.3)∆ |
| At the end of observation | 8.4 (5.3–10.6)" | 8.8 (7.2–10.7) | 10.6 (8.3–14.8)*∆ | |
# P < 0.05 when comparing the 1st and 2nd groups; ∆ P < 0.05 when comparing the 1st and 3rd groups; * P < 0.05 when comparing the 2nd and 3rd groups; " P < 0.05 when comparing the intragroup comparison (initially and at the end of the study). M is the mean value. AH: arterial hypertension; MS: metabolic syndrome; DM: diabetes mellitus; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; TI: time index; AI: area index; GHI: general hyperbaric index; BPV: blood pressure variability
The values of DBP TI at night in group 1 decreased to 40.0% (from 51.6 ± 8.4 to 32.0 ± 7.6; P = 0.001), in group 3 by 52.3% (from 68.4 ± 10.5 to 32.6 ± 8.2%; P = 0.00001). A decrease in BPV parameters in all groups indicated a persistent decrease in BP during the day in the course of therapy. The SBP/DBP dynamics variability in group 1—10.5%/11.8% (P < 0.05), in group 2—11.4% (P < 0.05)/8.3% (P = n/a), among representatives of the group 3—10.9% (P < 0.05)/9.4%(P = n/a).
The FC of CA and ACEI showed not only a pronounced antihypertensive effect, but also high metabolic neutrality, despite the initially exceeded values of glucose and creatinine in patients with MD (Table 4). In patients of group 1, a slight decrease in UA was detected, reaching statistically significant criteria (P = 0.03) without taking medications that affect the metabolism of UA. In contrast, a decrease in the hsCRP concentration was detected (P < 0.05) in patients of groups 2 and 3, which makes it possible for middle and/or high doses to influence the systemic inflammation activity (∆ = –12.8% in patients’ group 2 and ∆ = –11.2% in patients of group 3). Good tolerability of the combination was noted.
Dynamics of laboratory parameters of patients with hypertension on the background of ongoing therapy
| Indicator | Group 1АН without MSM ± m | Group 2AН and MSM ± m | Group 3AН and DМM ± m | |
|---|---|---|---|---|
| Total cholesterol, mmol/L | Initially | 4.85 ± 0.62 | 6.02 ± 0.61# | 5.96 ± 0.78∆ |
| At the end of observation | 4.77 ± 0.59" | 5,91 ± 0.58"# | 5,51 ± 0.60"∆ | |
| non-HDL, mmol/L | Initially | 3.89 ± 0.35 | 5.13 ± 0.31# | 4.78 ± 0.24∆ |
| At the end of observation | 3.42 ± 0.42 | 5.05 ± 0.46# | 4.65 ± 0.62∆ | |
| LDL, mmol/L | Initially | 3.09 ± 0.47 | 3.38 ± 0.44 | 3.71 ± 0.36 |
| At the end of observation | 3.07 ± 0.38" | 3.26 ± 0.51 | 3.42 ± 0.42 | |
| Creatinine, µmol/L | Initially | 74.57 ± 8.36 | 71.63 ± 6.80 | 94.93 ± 8.73∆* |
| At the end of observation | 70.82 ± 7.84" | 69.14 ± 5.53" | 87.64 ± 5.45"∆* | |
| Basal glucose, mmol/L | Initially | 5.07 ± 0.61 | 5.18 ± 0.73 | 7.42 ± 1.07∆* |
| At the end of observation | 5.01 ± 0.79 | 5.13 ± 0.85 | 6.89 ± 0.91"∆* | |
| НbА1с, % | Initially | - | - | 7.31 ± 0.9 |
| At the end of observation | - | - | 7.04 ± 0.5" | |
| Uric acid, µmol/L | Initially | 292.31 ± 62.37 | 412.75 ± 76.43# | 378.19 ± 58.28∆ |
| At the end of observation | 259.86 ± 43.22" | 401.53 ± 77.76# | 364.43 ± 63.55∆ | |
| hsCRP, mg/L | Initially | 1.81 ± 0.57 | 3.43 ± 0.93# | 3.50 ± 0.77∆ |
| At the end of observation | 1.69 ± 0.64 | 3.12 ± 0.61" | 3.11 ± 0.54" | |
# P < 0.05 comparing groups 1 and 2; ∆ P < 0.05 comparing groups 1 and 3; * P < 0.05 comparing groups 2 and 3; " P < 0.05 for intragroup comparison (initial and at the end of the study). M ± m, where M is the mean value, m is the standard deviation. AH: arterial hypertension; MS: metabolic syndrome; DM: diabetes mellitus; HDL: high-density lipoprotein; LDL: low-density lipoprotein; hsCRP: high-sensitivity C-reactive protein; НbА1с: the serum levels of glycated haemoglobin
It is known that in individuals with carbohydrate and purine metabolism disorders, AH is much more difficult than in those without metabolic abnormalities [3, 20]. The pathogenetic role of hyperglycemia, hyperinsulinemia and hyperuricemia in the AH formation was confirmed by modified SPBP and higher BP levels (both initially and at the end of the study) in patients of groups 2 and 3. The possibility to use FC of several antihypertensive medication groups allows one to simultaneously influence various parts of the AH pathogenesis, improve the pharmacokinetic profile of drug-drug interactions and provide effective organ protection.
The hyperglycemia and high BP relationship is so strong and well studied that in individuals with these disorders, the premature development of not only hypertension, but also coronary heart disease, heart failure, and cancer can be predicted [3, 20–22]. A consistently high blood glucose concentration has a direct toxic effect on the microvasculature and endothelium, leading to persistent muscle spasm formation and aggravating atherosclerotic vascular lesions (AVL). The AVL debut is also accelerated by hyperinsulinemia, which, by activating the proliferation of connective tissue and smooth muscle cells, contributes to thickening the vascular wall and its fibrosis. The hyperinsulinemia role in stimulating the SNS has also been proven, which is also inevitably associated with an increase in total vascular resistance and BP. At the same time, a simultaneous decrease the parasympathetic nervous system (PSNS) activity, under the influence of persistent hyperinsulinemia, increases the likelihood of rhythm disturbances [23], which was confirmed by the initially higher incidence of various arrhythmias in patients with MD.
The UA being the leading factor in cardiovascular risk is explained by the hyperuricemia’s ability to have a direct damaging effect on the endothelium and cause a pro-inflammatory response (local and systemic). With local hypoxia, phosphorylation of endothelial NO synthase slows down, which stimulates the tissue RAAS, leading to rapid progression of target organ damage and AVL [24]. A high UA level increases the SNS central nuclei activity [25], which was reflected in the BPV and HR parameters in patients of groups 2 and 3. When MD worsens together with decreased insulin sensitivity, the SAS influence increases [26], as evidenced by larger fluctuations in BP and shorter R-R intervals in patients with DM, significantly exceeding the physiological variability values.
The initially high creatinine concentration in the DM patients’ blood is also explained by the hypersympathicotonia influence. Sustained sympathetic stimulation of the heart and kidneys increases the renin secretion in the kidneys, which in turn activates the tissue RAAS [23, 26]. Therefore, taking into account the increased activity of the SNS and RAAS neurohumoral mechanisms, which play a significant role in disrupting local bioregulation systems, patients of groups 2 and 3 required AHT in maximum therapeutic doses to ensure nephroprotection in the first stages of its initiation. It can be assumed that the SAS long-term hyperactivity in uncontrolled AH is a “response” to tissue hypoxia, sufficient to maintain low-level systemic inflammation and aggravate MD. This was reflected in the hsCRP and UA initial values. Therefore, patients with pathogenetically substantiated MD were prescribed medications that simultaneously block both the RAAS manifestations, the SAS increased activity, and systemic inflammation symptoms. It has been experimentally proven that by reducing the RAAS activity and potentiating the kallikrein-kinin system effects, it is lipophilic ACEI (perindopril, ramipril) that largely reduces the SAS neurotransmitters secretion and affects the tonic/basal level of catecholamines, dopamine, and serotonin [27]. It is from this position that we considered the medicine FC with predominant renal elimination—lipophilic perindopril and vasoselective amlodipine—as basic AHT in patients with MD.
By the end of the first month of observation, the majority of study participants showed a significant BP decrease. This was the result of the cumulative effect of reducing the arterial vessel sensitivity to pressor agents, reducing total peripheral resistance and sustained vasodilation formation. After 3 months, all comparison groups showed an improvement in the main indicators of office BP measurements and ABPM indicators. The HR parameters and BPV had a clear positive trend. This was due not only to the ACEI/angiotension II receptor blockers (ARBs) pharmacological properties and a decrease in the SNS activity, but also to the baroreceptor reflex restoration, whose regulation normalisation leads to a decrease in BP fluctuations during a decrease in the overall hemodynamic load.
Patients with AH without MD achieved target BP values in a greater percentage and had a pronounced decline in average daily, daytime, nighttime SBP/DBP and their indexed indicators. The change in circadian BP rhythms in patients with DM was manifested by an increase in the modulation of the PSNS (vagus nerve) and provided a decrease in the variability of HR, nighttime SBP and DBP. However, this category of patients, despite a more significant reduction degree in global hyperbaric load, achieved target BP in a smaller percentage of cases. Difficulties of controlling BP in DM can be explained by several aspects: increased SBP and DBP variability, supported by hyperglycemia with frequent peak values [2, 26]; severe endothelial dysfunction, whose first evidence is the loss of the ability to regulate the ratio of synthesised biologically active vasoconstrictors (endothelin I, prostaglandins H2, G2, angiotensin II) and vasodilators (endothelium-dependent nitric oxide, prostacyclin, bradykinin) [27]; nocturnal hypersympathicotonia associated with hyperinsulinemia and low-intensity systemic inflammation of adipose tissue [22, 26]; damage to target organs, especially with the of autonomic neuropathy development [28], which determined the tactics viability of high AHT doses to achieve target BP values in this category of patients. In order to improve the BP profile, patients with DM at the stage of initiating AHT are likely to need prescribing ≥ 2 drugs with mandatory monitoring of the cardiometabolic RF influence.
A hsCRP concentration decrease at the end of the observation period in patients with MS and DM showed the ability of FC of the lipophilic ACEI and vasoselective CA to block the angiotensin II negative pro-oxidant effects, reduce the expression of many inflammatory cytokines (for example, COX-2, TNF-α, IL-6, TGF-β) and improve the immune system functions [29, 30]. It should be emphasised that the therapy did not require hospitalisation of any study participant due to the disease’s uncontrolled course and was accompanied by good medicine tolerance with a minimal incidence of side effects (6.9%).
In the next work, we will evaluate the effect of therapy depending on the obesity phenotype.
The identified DBP features determined the metabolic disorder contribution to the AH pathogenesis. In patients with MS and DM, there was a high SBP/DBP hyperbaric load, exceeded VBP values, and a predominant prognostically unfavourable BP profile with insufficient nocturnal reduction, indicating increased RAAS and SAS activity.
Our results added to the understanding of hypersympathicotonia and the role of MD in its maintenance. The SNS activation significance stimulated by hyperuricemia and hyperglycemia in the AH pathogenesis has been determined at the population level, which dictates the usefulness of mandatory antihyperuricemic medication prescription for asymptomatic hyperuricemia.
In order to achieve target BP values for persons with MD, at the stage of therapy initiation, it is advisable to choose the high therapeutic dosage tactics, and subsequently lower them with successful management of the main RFs’ influence.
Prescribing the lipophilic ACEI perindopril and the vasoselective CA amlodipine in the form of FC to patients with AH contributed to a persistent AHT effect, a decrease in the systemic inflammation activity and a significant nephroprotective effect, which favorably improves the life prognosis of patients with very high cardiometabolic risk.
ABPM: ambulatory blood pressure monitoring
ACEI: angiotensin-converting enzyme inhibitor
AH: arterial hypertension
AHT: antihypertensive therapy
AI: area index
AVL: atherosclerotic vascular lesions
BMI: body mass index
BP: blood pressure
BPV: blood pressure variability
CA: calcium antagonist
CH: cholesterol
DBP: diastolic blood pressure
DM: diabetes mellitus
FC: fixed combination
GHI: general hyperbaric index
HbA1c: glycated hemoglobin
HDL: high-density lipoprotein
HR: heart rate
hsCRP: high-sensitivity C-reactive protein
MD: metabolic disorders
MS: metabolic syndrome
PBP: pulse blood pressure
PSNS: parasympathetic nervous system
RAAS: renin-angiotensin-aldosterone system
RFs: risk factors
SAS: sympathoadrenal system
SBP: systolic blood pressure
SNS: sympathetic nervous system
TG: triglycerides
TI: time index
UA: uric acid
AAS: Conceptualization, Writing—review & editing. MAO: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. INV: Conceptualization, Investigation, Project administration, Validation, Writing—original draft, Writing—review & editing. MVT: Project administration, Writing—original draft. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was carried out taking into account international rules and ethical standards developed on the basis of the World Medical Association’s Declaration of Helsinki «Ethical Principles for Medical Research Involving Human Subjects». The study was approved by the Local Ethics Committee of Sechenov University (Extract from the Protocol of the 17–24 meeting dated 04.07.2024).
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 893
Download: 9
Times Cited: 0
