Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
2Institute of Clinical Medicine, National Research Lobachevsky State University of Nizhny Novgorod, 603022 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0002-2396-5054
Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0002-5961-9794
Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
Email: eliseevati@yandex.ru
ORCID: https://orcid.org/0000-0002-1769-3670
Affiliation:
3Medical Institute, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0002-4961-384X
Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0001-6153-6691
Affiliation:
3Medical Institute, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0001-7937-722X
Affiliation:
4Clinical Institute of Children’s Health named after N.F. Filatov, I.M. Sechenov First Moscow State Medical University (Sechenov University), 119991 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0003-0547-3686
Affiliation:
5Neurocognitive Research Laboratory, Kazan Federal University, 420000 Kazan, Russian Federation
ORCID: https://orcid.org/0000-0002-5582-5814
Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0002-8531-3174
Explor Med. 2025;6:1001338 DOl: https://doi.org/10.37349/emed.2025.1001338
Received: December 09, 2024 Accepted: May 06, 2025 Published: June 23, 2025
Academic Editor: Undurti N Das, UND Life Sciences, USA
Aim: To study the effect of body mass index (BMI) on interleukin (IL)-6 and IL-18 levels in children and adolescents with bronchial asthma (BA), taking into account the presence or absence of bronchial obstruction.
Methods: A single-center observational cross-sectional pilot study was conducted. Eighty-six patients with BA aged from 8 to 17 years were studied. Anthropometric and spirometric parameters and IL-6, IL-18 levels were assessed. The study participants were divided into 2 groups: 1—patients with normal body weight (BW), 2—overweight/obese.
Results: The serum levels of IL-6 and IL-18 were statistically significantly higher in overweight/obese patients than in normal BW patients, being 1.18 [0.10; 3.27] pg/mL vs. 0.52 [0.10; 1.34] pg/mL, P = 0.036 and 251.0 [207.0; 346.0] pg/mL vs. 208.0 [134.0; 293.0] pg/mL, P = 0.012, respectively. Statistically significant direct correlations of IL-6 and IL-18 with z BMI were obtained in the total group: R = 0.35, P = 0.001; R = 0.37, P = 0.002, respectively. In the overweight/obese group, IL-6 and IL-18 levels were statistically significantly higher in patients with obstruction [z forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) < –1.645 z] than in patients without obstruction (z FEV1/FVC > –1.645 z), respectively: 1.74 [1.10; 5.41] pg/mL vs. 0.59 [0.50; 1.48] pg/mL, P = 0.026 for IL-6 and 298.50 [207.00; 425.00] pg/mL vs. 234.50 [207.00; 300.00] pg/mL, P = 0.046 for IL-18.
Conclusions: In patients with BA and overweight/obese, but not in patients with BA and normal BW, the presence of bronchial obstruction is associated with higher serum levels of IL-6, IL-18. This may indicate the involvement of these ILs in the genesis of bronchial obstruction in patients with BA and overweight/obese.
The combination of bronchial asthma (BA) and obesity is associated with a decrease in the level of control asthma and resistance to treatment with inhaled glucocorticosteroids [1, 2], while the exact mechanisms of negative modification of the course of BA in overweight and obese children and adolescents have not yet been determined [3]. Currently, changes in the characteristics of T2-dependent inflammation, the pathogenetic basis of BA in children and adolescents, under the influence of excess adipose tissues are actively discussed [4, 5]. Obesity is characterized by an increase in the size and number of adipocytes and is accompanied by an increased synthesis of proinflammatory cytokines, including interleukin (IL)-6, IL-18 [6–10]. According to the results of the study by Pang et al. [7], serum levels of IL-6 and IL-18 demonstrate the most pronounced relationship with body weight (BW), and an increase in their systemic content influences the formation of somatic pathology in overweight/obesity.
The involvement of proinflammatory cytokines, including IL-6 and IL-18, in the pathogenesis of asthma has been the subject of active study for at least two decades [11–13]. Currently, the role of these cytokines in the pathogenesis of asthma is also being investigated in the combined setting of asthma and overweight/obesity.
IL-6 is a pleotropic cytokine that acts as a proinflammatory mediator and inducer of the acute phase response [14]. Considering the potential role of IL-6 in the pathogenesis of obesity and some endo-types of asthma, Akar-Ghibril et al. [15] and Permaul et al. [16] studied the levels of IL-6 in these conditions. Akar-Ghibril et al. [15] showed that serum IL-6 level increased significantly with increasing body mass index (BMI) in children, but no association was found between serum IL-6 levels with either the severity of asthma or a decrease in respiratory parameters characterizing bronchial patency. Adair et al. [17] highlight the significant association between IL-6, asthma, obesity, and metabolic dysfunction. However, in a recent study by Permaul et al. [16] reported that elevated serum IL-6 levels in children with BA were associated with decreased lung function and a higher risk of exacerbation, independent of the presence of obesity. Thus, the effect of BMI on the relationship between serum IL-6 and features of BA in children and adolescents remains a discussion.
The effect of overweight/obesity on IL-18 levels in patients with BA has also not been fully understood. It is known that IL-18 is a cytokine with proinflammatory properties that is involved in various inflammatory processes, including BA and obesity [18]. For example, Jung et al. [19] have demonstrated the relationship between IL-18 and obesity in children, and it has also been shown that IL-18 is increased in people with metabolic syndrome [20]. The studies by Xu et al. [21], Tanaka et al. [22], Kubysheva et al. [23] demonstrated the association of IL-18 with the pathogenesis of BA. However, Imaoka et al. [24] reported that IL-18 is not a marker of BA.
Thus, despite the available studies, the effect of BMI on the serum levels of IL-6 and IL-18 in children and adolescents with BA cannot be considered established, as well as the potential involvement of these cytokines in the pathogenesis of BA.
In this context, the aim of this study was to investigate the effect of BMI on IL-6 and IL-18 levels in children and adolescents with BA, taking into account the presence or absence of bronchial obstruction.
A single-center observational cross-sectional study was conducted.
The study was conducted in 2021–2024 at the Children’s City Clinical Hospital No. 1 in Nizhny Novgorod, Russia.
The study included patients with atopic asthma aged 8 to 17 years who were being treated for this condition in the outpatient clinic.
Family history of atopy (asthma, allergic rhinitis, conjunctivitis, atopic dermatitis, urticaria) was assessed. Sensitization to the main aeroallergens (allergens of house dust mite, cat, dog, pollen allergens) was assessed by in vivo (prick test) or in vitro (with the determination of specific IgE) methods. All children had partially controlled BA and were receiving basic therapy.
The inclusion criteria of the study were:
The diagnosis of asthma according to the current international conciliation documents (GINA, 2016–2024) [25].
The age of the patients is from 8 to 17 years old.
The exclusion criteria were:
Patients with BMI > +2.5 z.
The presence of acute infectious diseases and fever.
The presence of diabetes mellitus, autoimmune disease, primary immunodeficiency, cancer, atopic dermatitis, parasitic diseases.
Severe BA [25].
Systemic use of glucocorticoids.
The use of non-steroidal anti-inflammatory drugs, angiotensin-converting-enzyme (ACE) inhibitors, and drugs used in epilepsy.
The period of plant pollination.
All patients were assessed for basic anthropometric parameters. All measurements were taken without shoes, outer clothing, and headgear. Anthropometric parameters (height, BW, and BMI) were evaluated using tables developed by WHO, taking into account the sex and age of the patients [26].
Calculation of BMI: BMI = BW (kg)/height (m)2.
According to the BMI assessment data in this study, the children were divided into two groups: group 1: normal BW (BMI values from –2 z to +1 z), group 2: overweight, obese (BMI values above +1 z).
Body fat percentage (%BF): measured using a body composition monitor (Omron BF 214, Japan).
Waist circumference (WC): measurements were carried out at the end of normal exhalation using a flexible tape on a circle equidistant between the upper border of the iliac crest and the lower edge of the rib.
The ratio of WC to height was calculated using the formula: WC/Height.
Spirometric studies were performed using the spirometer MasterScreen Pneumo (Erich Jaeger GmbH, Germany). The following parameters were evaluated in the analysis of the spirometry data:
Forced vital capacity (FVC) of the lungs.
Forced expiratory volume in one second (FEV1).
FEV1/FVC ratio, which is the main spirometry parameter used in the diagnosis of obstructive disease.
In addition, z FVC, z FEV1, and z FEV1/FVC were calculated using the Global Lung Function Initiative calculator, developed with the support of the European Respiratory Society [27].
We define the presence of airway obstruction at the time of the examination as z FEV1/FVC less than –1.645 z [28].
Serum levels of IL-6 and IL-18 were determined using Interleukin-6-ELISA-Best test systems manufactured by Vector-Best JSC, Interleukin-18-ELISA-Best manufactured by Vector-Best JSC, Russia, using an automated enzyme immunoassay analyzer Radim Alisei Q.S., Radim Diagnostics, Inc., Italy. The detection sensitivity of IL-6 was 0.1 pg/mL, with a range of 0–90 pg/mL. The detection sensitivity of IL-18 was 0.5 pg/mL, with a range of 0–800 pg/mL. All blood samples were taken in the morning, before breakfast.
The study was a pilot study, and the sample size was not calculated. Analysis was performed using Statgraphics Centurion v.16 (Statgraphics Technologies, Inc., The Plains, Virginia, USA). Quantitative indicators were evaluated for compliance with the normal distribution. Data are presented as Me [Q1; Q3], where Me is the median, [Q1; Q3] is 1 and 3 quartiles due to a distribution different from normal. The Mann-Whitney criterion was used to compare quantitative variables in two independent groups. Correlation analysis was performed using Spearman’s correlation coefficient. The Chi-square criterion was used to compare qualitative variables. The differences were considered statistically significant at P < 0.05.
In this study, 86 patients aged 8 to 17 years were examined, of whom 41.9% (36/86) were overweight/obese. There were no statistically significant differences between normal weight and overweight/obese patients according to sex and age (Table 1). The parameters z height, z BMI, %BF, and WC/height were statistically significantly higher in overweight/obese patients, P < 0.05. z FVC was statistically significantly higher, P = 0.012, and z FEV1/FVC was statistically significantly lower in overweight/obese group, P = 0.035.
Clinical characteristics of patients, spirometric parameters
| Parameters | Normal weight (n = 50) | Overweight/obese (n = 36) | P-value |
|---|---|---|---|
| Age, years | 13.5 [10.0; 15.0] | 13.0 [11.0; 16.0] | 0.865 |
| Boys, n = 66 | 76.0% (38/50) | 77.8% (28/36) | 0.669* |
| z Height | 0.30 [–0.45; 0.97] | 1.21 [0.58; 1.84] | < 0.001 |
| z BMI | –0.09 [–0.43; 0.53] | 1.40 [1.22; 1.92] | < 0.001 |
| %BF, % | 18.2 [11.7; 22.7] | 26.3 [21.5; 33.1] | 0.002 |
| WC/height | 0.43 [0.42; 0.45] | 0.55 [0.49; 0.58] | < 0.001 |
| z FVC | 0.96 [0.19; 1.74] | 1.56 [0.85; 2.23] | 0.012 |
| z FEV1/FVC | –0.89 [–2.07; 0.10] | –1.56 [–2.13; –0.81] | 0.035 |
| IgE, IU/mL | 261.8 ± 242.5 | 173.0 ± 115.8 | > 0.05 |
P < 0.05 represents a significant difference in results. * chi-square test. BMI: body mass index; %BF: body fat percentage; WC: waist circumference; FVC: forced vital capacity; FEV1: forced expiratory volume in one second
The serum levels of IL-6 and IL-18 were statistically significantly higher in overweight/obese patients than in normal-weight patients: 1.18 [0.10; 3.27] pg/mL vs. 0.52 [0.10; 1.34] pg/mL, P = 0.036 and 251.0 [207.0; 346.0] pg/mL vs. 208.0 [134.0; 293.0] pg/mL, P = 0.012, while the median values of the indicators were within the reference values (Figure 1).
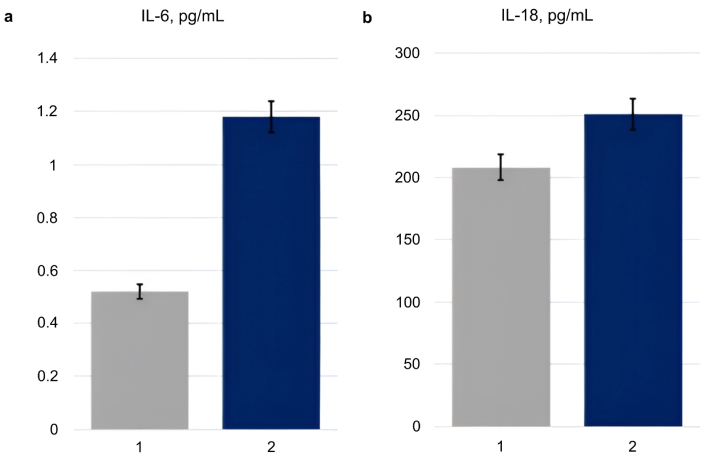
The serum levels of interleukin (IL)-6 and IL-18 in the normal-weight (1) and overweight/obese (2) patients. (a) The level of IL-6 (pg/mL) in study participants with different body weights (1: normal weight, n = 50; 2: overweight/obese, n = 36). P = 0.036; (b) the level of IL-18 (pg/mL) in study participants with different body mass index (BMI) (1: normal weight, n = 50; 2: overweight/obese, n = 36). P = 0.012
Statistically significant direct correlations of IL-6 and IL-18 levels were obtained between z BMI, %BF, WC/height, all P < 0.05 (Table 2).
Correlations between the levels of IL-6, IL-18 and anthropometric parameters, parameters of body composition (all participants, n = 86)
| Parameters | IL-6, pg/mL | IL-18, pg/mL | ||
|---|---|---|---|---|
| R | P-value | R | P-value | |
| z BMI | 0.35 | 0.001 | 0.37 | 0.002 |
| %BF, % | 0.40 | 0.016 | 0.37 | 0.047 |
| WC/height | 0.35 | 0.025 | 0.33 | 0.056 |
P < 0.05 indicates a significant difference in results. R: correlation coefficient. IL: interleukin; BMI: body mass index; %BF: body fat percentage; WC: waist circumference
In the overweight/obese group, IL-6 and IL-18 levels were statistically significantly higher in patients with obstruction (z FEV1/FVC < –1.645 z) than in patients without obstruction (z FEV1/FVC > –1.645 z), respectively: 1.74 [1.10; 5.41] pg/mL vs. 0.59 [0.50; 1.48] pg/mL, P = 0.026 for IL-6 and 298.50 [207.00; 425.00] pg/mL vs. 234.50 [207.00; 300.00] pg/mL, P = 0.046 for IL-18 (Figures 2 and 3). In normal weight study participants, both in the presence and absence of obstruction, serum levels of IL-6 and IL-18 were comparable (P = 0.250, P = 0.898, respectively).
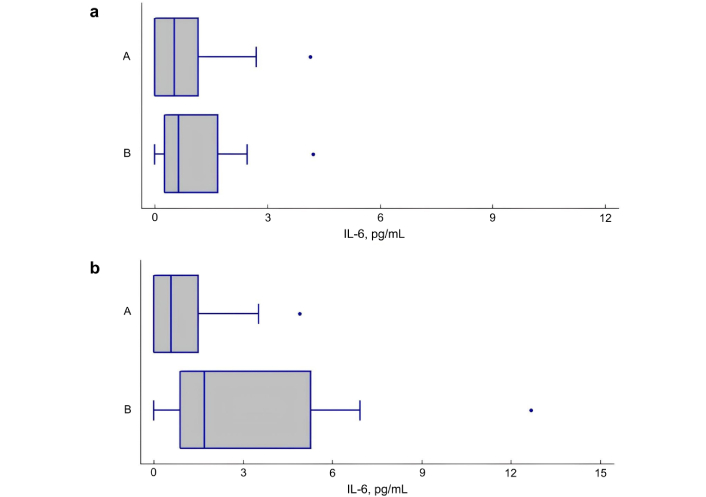
The serum levels of interleukin (IL)-6 in patients with asthma with and without airway obstruction. (a) The level of IL-6 (pg/mL) in patients with normal body weight, depending on the presence (B) or absence (A) of obstruction. P = 0.250; (b) The level of IL-6 (pg/mL) in overweight/obese patients, depending on the presence (B) or absence (A) of obstruction. P = 0.026. A: obstruction absence [z forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) > –1.645 z]; B: obstruction presence (z FEV1/FVC < –1.645 z)
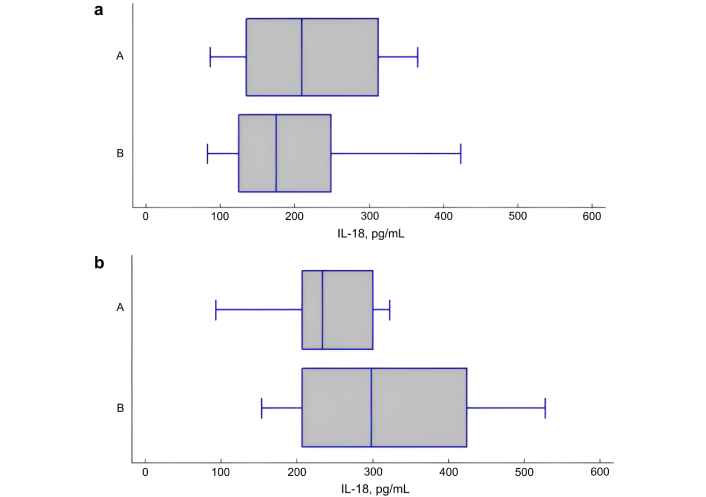
The serum levels of interleukin (IL)-18 in patients with asthma with and without airway obstruction. (a) The level of IL-18 (pg/mL) in patients with normal body weight, depending on the presence (B) or absence (A) of obstruction. P = 0.898; (b) The level of IL-18 (pg/mL) in overweight/obese patients, depending on the presence (B) or absence (A) of obstruction. P = 0.046. A: obstruction absence [z forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) > –1.645 z]; B: obstruction presence (z FEV1/FVC < –1.645 z)
To the best of our knowledge, this study was the first to investigate the effect of BMI on serum levels of IL-6 and IL-18 in children and adolescents with BA. Statistically significant direct correlations of IL-6 and IL-18 with z BMI were obtained in the general group: R = 0.35, P = 0.001 and R = 0.37, P = 0.002, respectively.
The statistically significantly higher levels of serum IL-6 and IL-18 in patients with BA combined with overweight/obesity found in this study, compared with patients with BA of normal weight, probably reflect low-grade systemic inflammation generated by excess adipose tissue. Increased levels of IL-6 in children with asthma and obesity were noted in the work of Akar-Ghibril et al. [15] and Permaul et al. [16], which is consistent with our data. The studies by Sindhu et al. [29] demonstrated that obesity is a positive modulator of IL-6R and IL-6 expression in the adipose tissue, which might be a contributory mechanism to induce metabolic inflammation. Elevated IL-18 levels in adult patients with asthma and obesity have been reported in the work of Bantulà et al. [30]. We did not find any studies on the association of IL-18 with BA and obesity in children.
In our study, IL-6 and IL-18 levels depended on the presence or absence of obstruction, which was diagnosed by the level of spirometric parameters: z FEV1/FVC less than –1.645 z. In the overweight/obese group, IL-6 and IL-18 concentrations were statistically significantly higher in patients with obstruction (z FEV1/FVC < –1.645 z) than in patients without obstruction (z FEV1/FVC > –1.645 z), respectively: 1.74 [1.10; 5.41] pg/mL vs. 0.59 [0.50; 1.48] pg/mL, P = 0.026 for IL-6 and 298.50 [207.00; 425.00] pg/mL vs. 234.50 [207.00; 300.00] pg/mL, P = 0.046 for IL-18, respectively. In normal weight participants, serum IL-6 and IL-18 levels were comparable in the presence and absence of obstruction (P = 0.250 and P = 0.898, respectively). We did not find any studies on IL-6 and IL-18 levels in the presence of obstruction in children with BA and overweight/obesity.
Thus, in patients with BA in combination with overweight/obesity but not in patients with BA and normal BW, the presence of bronchial obstruction is associated with higher serum levels of IL-6, IL-18. This may indicate the involvement of these ILs in the genesis of bronchial obstruction in patients with BA in combination with overweight/obesity.
Unfortunately, there were some limitations in our study. First, the number of cases (86) included in our study was limited. Second, it was a cross-sectional study. Third, we did not take into account Tanner stage and potential markers of metabolic syndrome (e.g., uric acid). However, our study is ongoing, and these factors will be taken into account in the future.
%BF: body fat percentage
BA: bronchial asthma
BMI: body mass index
BW: body weight
FEV1: forced expiratory volume in one second
FVC: forced vital capacity
IL: interleukin
WC: waist circumference
RNK: Investigation, Writing—original draft, Writing—review & editing. EVT: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. TIE: Conceptualization, Investigation, Writing—review & editing. DYO: Validation, Writing—review & editing, Supervision. SVK: Writing—review & editing. MAK: Investigation, Writing—original draft. NAG: Validation, Writing—review & editing, Supervision. NIK: Writing—review & editing. OVK: Conceptualization, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was performed in accordance with the Helsinki Declaration (2013) and approved by the Ethics Committee of the Privolzhsky Research Medical University (Protocol No. 8 dated 27.05.2022). Informed consent was obtained from patients aged 15 to 17 years and from the parents of patients under the age of 15 in accordance with Federal Law No. 323 dated 11/21/2011, “Fundamentals of legislation of the Russian Federation on the protection of public health”.
Informed consent to participate in the study was obtained from patients aged 15 to 17 years and from the parents of patients under the age of 15.
Not applicable.
The data used to support the findings of this study are available from the corresponding author upon request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1297
Download: 19
Times Cited: 0
