Diagnostic and prognostic role of liquid biopsy in non-small cell lung cancer: evaluation of circulating biomarkers
Lung cancer is still one of the main causes of cancer-related death, together with prostate and colorectal cancers in males and breast and colorectal cancers in females. The prognosis for non-small
[...] Read more.
Lung cancer is still one of the main causes of cancer-related death, together with prostate and colorectal cancers in males and breast and colorectal cancers in females. The prognosis for non-small cell lung cancer (NSCLC) is strictly dependent on feasibility of a complete surgical resection of the tumor at diagnosis. Since surgery is indicated only in early stages tumors, it is necessary to anticipate the timing of diagnosis in clinical practice. In the diagnostic and therapeutic pathway for NSCLC, sampling of neoplastic tissue is usually obtained using invasive methods that are not free from disadvantages and complications. A valid alternative to the standard biopsy is the liquid biopsy (LB), that is, the analysis of samples from peripheral blood, urine, and other biological fluids, with a simple and non-invasive collection. In particular, it is possible to detect in the blood different tumor derivatives, such as cell-free DNA (cfDNA) with its subtype circulating tumor DNA (ctDNA), cell-free RNA (cfRNA), and circulating tumor cells (CTCs). Plasma-based testing seems to have several advantages over tumor tissue biopsy; firstly, it reduces medical costs, risk of complications related to invasive procedures, and turnaround times; moreover, the analysis of genes alteration, such as EGFR, ALK, ROS1, and BRAF is faster and safer with this method, compared to tissue biopsy. Despite all these advantages, the evidences in literatures indicate that assays performed on liquid biopsies have a low sensitivity, making them unsuitable for screening in lung cancer at the current state. This is caused by lack of standardization in sampling and preparation of specimen and by the low concentration of biomarkers in the bloodstream. Instead, routinely use of LB should be preferred in revaluation of patients with advanced NSCLC resistant to chemotherapy, due to onset of new mutations.
Giovanni Vicidomini ... Mario Santini
Lung cancer is still one of the main causes of cancer-related death, together with prostate and colorectal cancers in males and breast and colorectal cancers in females. The prognosis for non-small cell lung cancer (NSCLC) is strictly dependent on feasibility of a complete surgical resection of the tumor at diagnosis. Since surgery is indicated only in early stages tumors, it is necessary to anticipate the timing of diagnosis in clinical practice. In the diagnostic and therapeutic pathway for NSCLC, sampling of neoplastic tissue is usually obtained using invasive methods that are not free from disadvantages and complications. A valid alternative to the standard biopsy is the liquid biopsy (LB), that is, the analysis of samples from peripheral blood, urine, and other biological fluids, with a simple and non-invasive collection. In particular, it is possible to detect in the blood different tumor derivatives, such as cell-free DNA (cfDNA) with its subtype circulating tumor DNA (ctDNA), cell-free RNA (cfRNA), and circulating tumor cells (CTCs). Plasma-based testing seems to have several advantages over tumor tissue biopsy; firstly, it reduces medical costs, risk of complications related to invasive procedures, and turnaround times; moreover, the analysis of genes alteration, such as EGFR, ALK, ROS1, and BRAF is faster and safer with this method, compared to tissue biopsy. Despite all these advantages, the evidences in literatures indicate that assays performed on liquid biopsies have a low sensitivity, making them unsuitable for screening in lung cancer at the current state. This is caused by lack of standardization in sampling and preparation of specimen and by the low concentration of biomarkers in the bloodstream. Instead, routinely use of LB should be preferred in revaluation of patients with advanced NSCLC resistant to chemotherapy, due to onset of new mutations.
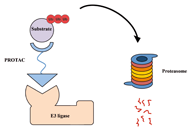 Novel approaches for the rational design of PROTAC linkersOpen AccessReviewProteolysis targeting chimeras (PROTACs) represent a promising class of hetero-bivalent molecules that facilitate ubiquitination of a target protein by simultaneously binding and bringing together b [...] Read more.Almaz Zagidullin ... Emil BulatovPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:381–390
Novel approaches for the rational design of PROTAC linkersOpen AccessReviewProteolysis targeting chimeras (PROTACs) represent a promising class of hetero-bivalent molecules that facilitate ubiquitination of a target protein by simultaneously binding and bringing together b [...] Read more.Almaz Zagidullin ... Emil BulatovPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:381–390 Potential of guggulsterone, a farnesoid X receptor antagonist, in the prevention and treatment of cancerOpen AccessReviewCancer is one of the most dreadful diseases in the world with a mortality of 9.6 million annually. Despite the advances in diagnosis and treatment during the last couple of decades, it still remains [...] Read more.Sosmitha Girisa ... Ajaikumar B. KunnumakkaraPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:313–342
Potential of guggulsterone, a farnesoid X receptor antagonist, in the prevention and treatment of cancerOpen AccessReviewCancer is one of the most dreadful diseases in the world with a mortality of 9.6 million annually. Despite the advances in diagnosis and treatment during the last couple of decades, it still remains [...] Read more.Sosmitha Girisa ... Ajaikumar B. KunnumakkaraPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:313–342 Pseudoprogression in lung cancer: a case reportOpen AccessCase ReportImmunotherapy dramatically changed the management of several malignancies including non-small cell lung cancer (NSCLC). Since immune checkpoint inhibitors have a different mechanism of action from c [...] Read more.Giulia Meoni ... Angela Stefania RibeccoPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:372–380
Pseudoprogression in lung cancer: a case reportOpen AccessCase ReportImmunotherapy dramatically changed the management of several malignancies including non-small cell lung cancer (NSCLC). Since immune checkpoint inhibitors have a different mechanism of action from c [...] Read more.Giulia Meoni ... Angela Stefania RibeccoPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:372–380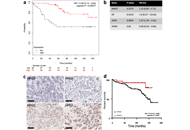 Downregulation of 15-hydroxyprostaglandin dehydrogenase during acquired tamoxifen resistance and association with poor prognosis in ERα-positive breast cancerOpen AccessOriginal ArticleAim: Tamoxifen (TAM) resistance remains a clinical issue in breast cancer. The authors previously reported that 15-hydroxyprostaglandin dehydrogenase (HPGD) was significantly downregulated in tamox [...] Read more.Milene Volpato ... Valerie SpeirsPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:355–371
Downregulation of 15-hydroxyprostaglandin dehydrogenase during acquired tamoxifen resistance and association with poor prognosis in ERα-positive breast cancerOpen AccessOriginal ArticleAim: Tamoxifen (TAM) resistance remains a clinical issue in breast cancer. The authors previously reported that 15-hydroxyprostaglandin dehydrogenase (HPGD) was significantly downregulated in tamox [...] Read more.Milene Volpato ... Valerie SpeirsPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:355–371 Current strategies for the design of PROTAC linkers: a critical reviewOpen AccessReviewPROteolysis TArgeting Chimeras (PROTACs) are heterobifunctional molecules consisting of two ligands; an “anchor” to bind to an E3 ubiquitin ligase and a “warhead” [...] Read more.Robert I. Troup ... Matthias G. J. BaudPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:273–312
Current strategies for the design of PROTAC linkers: a critical reviewOpen AccessReviewPROteolysis TArgeting Chimeras (PROTACs) are heterobifunctional molecules consisting of two ligands; an “anchor” to bind to an E3 ubiquitin ligase and a “warhead” [...] Read more.Robert I. Troup ... Matthias G. J. BaudPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:273–312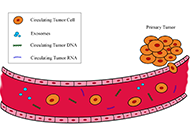 Diagnostic and prognostic role of liquid biopsy in non-small cell lung cancer: evaluation of circulating biomarkersOpen AccessReviewLung cancer is still one of the main causes of cancer-related death, together with prostate and colorectal cancers in males and breast and colorectal cancers in females. The prognosis for non-small [...] Read more.Giovanni Vicidomini ... Mario SantiniPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:343–354
Diagnostic and prognostic role of liquid biopsy in non-small cell lung cancer: evaluation of circulating biomarkersOpen AccessReviewLung cancer is still one of the main causes of cancer-related death, together with prostate and colorectal cancers in males and breast and colorectal cancers in females. The prognosis for non-small [...] Read more.Giovanni Vicidomini ... Mario SantiniPublished: October 30, 2020 Explor Target Antitumor Ther. 2020;1:343–354