Fibrosis and Hepatobiliary Cancer
Guest Editors
Prof. Fabio Marra E-Mail
University of Florence, Florence, Italy
Research Keywords: Cellular and molecular mechanisms of hepatic inflammation and fibrogenesis, pathophysiology of nonalcoholic steatohepatitis, hepatobiliary neoplasia, non-invasive evaluation of liver fibrosis
Dr. Chiara Raggi E-Mail
Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy
Research Keywords: Hepatobiliary tumor, cholangiocarcinoma, cancer stem cells, tumor associated macrophages, tumor metabolism
About the Special lssue
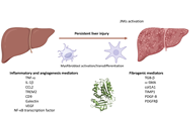
Hepatic fibrosis is a pathophysiological outcome of chronic liver injury characterized by progressive accumulation of extracellular matrix proteins, leading to a profound alteration of the physiological architecture of the liver. It represents a dynamic process that involves cross-talk between parenchymal cells (hepatocytes), hepatic stellate cells, sinusoidal endothelial cells and both resident and infiltrating immune cells. Liver fibrosis is a major health problem that still lacks effective therapeutic strategies. Therefore, understanding the mechanisms underlying this process is crucial for translating basic research into new clinical therapies.
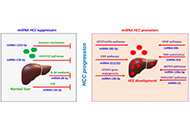
Moreover, advanced liver fibrosis results in cirrhosis, portal hypertension, and liver failure and often requires liver transplantation. Importantly, advanced liver fibrosis and cirrhosis are also major risk factors for hepatobiliary cancer. Indeed, more than 80% of hepatocellular carcinomas (HCC) develop in fibrotic or cirrhotic livers, suggesting an important role of liver fibrosis in determining a premalignant hepatic environment. Likewise, cholangiocarcinoma (CCA) is characterized by a strong desmoplasia that typically occurs in response to the tumor, suggesting a key role of cancer-associated fibroblasts and fibrosis in its tumor microenvironment. Better understanding of the role of myofibroblasts in HCC and CCA development and progression may provide the basis to target these cells for tumor prevention or therapy. This Special Issue will provide insights into basic mechanisms that influence liver fibrosis development by exploring the intercellular interaction between liver cells and myofibroblasts and highlighting the importance of targeting them as a novel therapeutic strategy
Keywords: Inflammation, NASH, NAFLD, HCC, CCA, hepatic stellate cells, cancer associated fibroblasts
Published Articles