-
 Special Issue Topic
Special Issue TopicRecent Advances in Neurochemistry and Genetics of Late-onset Alzheimer's Disease
Submission Deadline: December 31, 2022Guest Editor
Dr. Vladimir J. Balcar E-Mail
Honorary Associate Professor, Faculty of Medicine and Health, The University of Sydney, Australia
Research Keywords: glutamate; GABA; neurotransmitter transporters; genetics of neurodegenerative diseases
About the Special Issue
Several genes have been identified as “genes of interest” (GoI) in Alzheimer’s disease (AD). Recent Genome-wide Association Studies (GWAS) confirmed many of the existing GoI; newly identified AD-related loci, however, still await additional verifications in terms of potential significance and impacted signals/mechanisms[1]. Some of the most important GoI mutations were first identified as genetic correlates of the early-onset form of AD (EOAD) thus accounting for only a small fraction of the total number of AD cases. Translating similar findings to the late-onset Alzheimer’s disease (LOAD) may pose a challenge; LOAD is a “multifactorial” condition influenced by both life-style and genetics, probably representing a group of diseases with distinct causes and mechanisms. It is, therefore, remarkable that several new GoI have in recent years been found to be associated directly with LOAD[2]. The studies used case-association (rather than GWAS) approach targeting well-defined groups of patients and controls[2]. Descriptions of related neurochemical mechanisms and explanations how they might relate to the loss of neurons in AD have been attempted but remain, at best, speculative. Additional research is needed, aimed at LOAD.
The proposed thematic issue invites papers:
(1) presenting evidence of novel GoI and molecules of interest in AD with a focus on LOAD.
(2) offering novel explanations of how the variants of existing GoI might influence the risk of LOAD.
Genetics is not limited to analysing DNA and/or identifying new GoI; given the very recent developments in the research on COVID-19 (SARS-CoV-2), the issue also invites studies.(3) showing how neuroinvasive viruses could alter DNA structure or DNA repair and/or gene translation, influencing the long-term risk of AD[3,4].
(4) bringing any new ideas and/or data on how variations in genes and changes in the molecules that they encode may impact the risk of AD.
References
1. Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022;54:412-36.
2. Šerý O, Zeman T, Sheardová K, Vyhnálek M, Marková H, Laczó J, et al. Six genetically linked mutations in the CD36 gene significantly delay the onset of Alzheimer's disease. Sci Rep. 2022;12:10994.
3. Tirozzi A, Santonastaso F, de Gaetano G, Iacoviello L, Gialluisi A. Does COVID-19 increase the risk of neuropsychiatric sequelae? Evidence from a mendelian randomization approach. World J Psychiatry. 2022;12:536-40.
4. Reiken S, Sittenfeld L, Dridi H, Liu Y, Liu XP, Marks AR. Alzheimer's-like signaling in brains of COVID-19 patients. Alzheimers Dement. 2022;18:955-65.Keywords: single nucleotide polymorphism; SNP; case-association study; LOAD; late-onset Alzheimer’s Disease; genetics of Alzheimer’s Disease; genetic risk of Alzheimer’s
Call for Papers
Published Articles
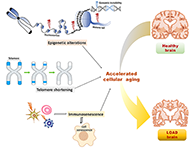 Epigenetic and non-epigenetic mechanisms in the accelerated cellular aging in late-onset Alzheimer’s diseaseOpen AccessReviewLate-onset Alzheimer's disease (LOAD) is the most common form of Alzheimer's disease (AD) and its risk increases exponentially with aging. The incidence of LOAD is reported to [...] Read more.Kajal Rawat, Prathiba GarlapallyPublished: April 28, 2023 Explor Neuroprot Ther. 2023;3:105–119
Epigenetic and non-epigenetic mechanisms in the accelerated cellular aging in late-onset Alzheimer’s diseaseOpen AccessReviewLate-onset Alzheimer's disease (LOAD) is the most common form of Alzheimer's disease (AD) and its risk increases exponentially with aging. The incidence of LOAD is reported to [...] Read more.Kajal Rawat, Prathiba GarlapallyPublished: April 28, 2023 Explor Neuroprot Ther. 2023;3:105–119
DOI: https://doi.org/10.37349/ent.2023.00040 -
-
Ongoing Special Issues
-
Completed Special Issues