-
 Special Issue Topic
Special Issue TopicBiomarkers in Amyotrophic Lateral Sclerosis
Submission Deadline: September 30, 2022Guest Editor
Prof. Christopher A. Shaw E-Mail
Professor at The University of British Columbia, Vancouver, Canada
Research Keywords: amyotrophic lateral sclerosis; neuroplasticity; neuropathology; ALS-parkinsonism dementia complex (ALS-PDC)
About the Special Issue
Almost 125 years after Charcot first described and named amyotropic lateral sclerosis (ALS), the disease remains in large measure a mystery. For example, we know that motor neurons are the key targets of the disease process, but the field is still vague on the roles of the other types of neurons that are impacted, not least the roles of the various types of glial cells. We know that most cases are sporadic, that is, of unknown origin, but with strong suspicions that various toxic substances play major roles, but cannot definitively settle on any of them as the main players. We know some gene mutations that cause motor neuron loss, but are only at the beginning of understanding the role of the so-called “junk” DNA, that is the non-coding regions. We know virtually nothing about the interactions of DNA and toxins leading to disease. A lot of the biochemistry we think we know arises from a few gene models in rodents, thus giving biochemical pathways that may, or may not, be relevant to human disease. In fact, we have very few, if any, really good models of either genetic causality leading to motor neuron degeneration or of toxins that might do the same.
What we can now say with some certainty is that ALS is almost certainly a “spectrum” disorder that has multiple pathways that are involved at different times and that other non-motor systems in the CNS are often affected.
Given the later in particular, fairly intensive research has yet to come up with effective long-term treatments to halt or even significantly slow disease progression. This is true as well for the other major age-dependent neurological disorders, Alzheimer’s and Parkinson’s diseases. It is, however, the speed of the disease that most impedes the hunt for effective therapies: from clinical diagnosis to death is typically 3 to 5 years, a period characterized by cascading failure within the nervous system of the patients. Further, all of us in the field have seen the various “omics” studies which suggest dozens if not hundreds of genes/single nucleotide polymorphisms, metabolic pathways, and proteins over- and under-represented at the end stage. Added to this difficulty, we have no way of currently knowing which of these elements are part of a causal process, which ones are merely “bystanders”, and which might even be an attempt by the nervous system to compensate for the damage.
The clearest take away message from such studies is that there is unlikely to be any therapeutic strategy to effectively cope with such multiple abnormalities and that the only realistic solution is to find the earliest stages of the disease, whenever they occur, and try to prevent the stage where “cascading failure” is out of control. It will be obvious that we cannot do this with an approach such as the Framingham study for cardiovascular diseases: ALS remains too rare to have sufficient numbers.
While there are many researchers who continue to hope for a “universal” toxin, a common gene, or a converging pathway between the two, there is little or nothing in the current literature that suggests that this will turn out to be true. What remains, apart from luck, is the need for a deliberate effort to find early phase biomarkers that will alert us to the beginning of the various processes leading to motor neuron loss and the expression of ALS.
Such biomarkers will certainly have to be found peripherally since invasive procedures are unlikely to be tolerated, let alone affordable, by most future victims.
This volume is designed to explore possible biomarkers in ALS and how they might be used to identify key early stages in the overlapping diseases that fall under the rubric of ALS. For me, this is the crucial stage in any attempt “to make ALS a treatable disorder.” If we succeed, there may be a path forward, even in the face of likely multiple mechanisms leading to motor neuron death. If we don’t, then, simply put, we remain where we are with meagre treatment options.
The goal of the book is to determine where biomarker research in ALS is at this point in time and if it has any hope to change the treatment options for the better for the victims.
Keywords: spectrum disorder; cascading failure; gene-toxin interactions; toxins; DNA interactions; therapeutic failure
Call for Papers
Published Articles
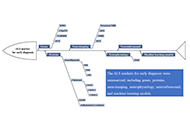 Breaking the amyotrophic lateral sclerosis early diagnostic barrier: the promise of general markersOpen AccessReviewAmyotrophic lateral sclerosis (ALS) is a severe neurodegenerative disease that is associated with selective and progressive loss of motor neurons. As a consequence, the symptoms of ALS are muscle cr [...] Read more.Yizhou Lu ... Qinming ZhouPublished: December 27, 2023 Explor Neuroprot Ther. 2023;3:497–512
Breaking the amyotrophic lateral sclerosis early diagnostic barrier: the promise of general markersOpen AccessReviewAmyotrophic lateral sclerosis (ALS) is a severe neurodegenerative disease that is associated with selective and progressive loss of motor neurons. As a consequence, the symptoms of ALS are muscle cr [...] Read more.Yizhou Lu ... Qinming ZhouPublished: December 27, 2023 Explor Neuroprot Ther. 2023;3:497–512
DOI: https://doi.org/10.37349/ent.2023.00065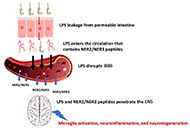 Unique cerebrospinal fluid peptides: potential amyotrophic lateral sclerosis biomarkers and etiological factorsOpen AccessOriginal ArticleAim: Amyotrophic lateral sclerosis (ALS) is a progressive disease of unknown etiology, characterized by degeneration of motoneurons and skeletal muscle strength decline that progressively evolves [...] Read more.Uri Wormser ... Yoram FinkelsteinPublished: December 12, 2023 Explor Neuroprot Ther. 2023;3:435–445
Unique cerebrospinal fluid peptides: potential amyotrophic lateral sclerosis biomarkers and etiological factorsOpen AccessOriginal ArticleAim: Amyotrophic lateral sclerosis (ALS) is a progressive disease of unknown etiology, characterized by degeneration of motoneurons and skeletal muscle strength decline that progressively evolves [...] Read more.Uri Wormser ... Yoram FinkelsteinPublished: December 12, 2023 Explor Neuroprot Ther. 2023;3:435–445
DOI: https://doi.org/10.37349/ent.2023.00060 Retracted: Diffusion magnetic resonance imaging-based surrogate marker in amyotrophic lateral sclerosisOpen AccessReviewAmyotrophic lateral sclerosis (ALS) is the most prevalent type of motor neuron disease (MND) and is diagnosed with a delay from the first appearance of symptoms. Surrogate markers that may be used t [...] Read more.Yuya SaitoPublished: August 25, 2023 Explor Neuroprot Ther. 2023;3:186–206
Retracted: Diffusion magnetic resonance imaging-based surrogate marker in amyotrophic lateral sclerosisOpen AccessReviewAmyotrophic lateral sclerosis (ALS) is the most prevalent type of motor neuron disease (MND) and is diagnosed with a delay from the first appearance of symptoms. Surrogate markers that may be used t [...] Read more.Yuya SaitoPublished: August 25, 2023 Explor Neuroprot Ther. 2023;3:186–206
DOI: https://doi.org/10.37349/ent.2023.00047 Muscle fatigue and exercise-related biomarkers in amyotrophic lateral sclerosisOpen AccessReviewAmyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder affecting motor neurons. The complex etiopathogenetic mechanism of ALS can lead to extensive alterations, including co [...] Read more.Francesca Bianchi ... Gabriele SicilianoPublished: June 30, 2023 Explor Neuroprot Ther. 2023;3:164–176
Muscle fatigue and exercise-related biomarkers in amyotrophic lateral sclerosisOpen AccessReviewAmyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder affecting motor neurons. The complex etiopathogenetic mechanism of ALS can lead to extensive alterations, including co [...] Read more.Francesca Bianchi ... Gabriele SicilianoPublished: June 30, 2023 Explor Neuroprot Ther. 2023;3:164–176
DOI: https://doi.org/10.37349/ent.2023.00045 -
-
Ongoing Special Issues
-
Completed Special Issues