Cancer Immunotherapy and Tumor Microenvironment
Guest Editors
Dr. Fathia Mami-Chouaib E-Mail
INSERM UMR 1186, Integrative Tumour Immunology and Immunotherapy, Gustave Roussy, Fac. de Médecine - Univ. Paris-Sud, Université Paris-Saclay, 94805, Villejuif, France
Research Keywords: Onco-immunology; cancer immunotherapy; cancer vaccine; T lymphocytes; CTL; TRM
Dr. Salem Chouaib E-Mail
INSERM UMR 1186, Integrative Tumour Immunology and Immunotherapy, Gustave Roussy, Fac. de Médecine - Univ. Paris-Sud, Université Paris-Saclay, 94805, Villejuif, France; Thumbay Research Institute for Precision Medicine, Gulf Medical University, Ajman, United Arab Emirates
Research Keywords: Onco-immunology; tumor microenvironment; hypoxia; EMT; tumor immune escape
About the Special lssue
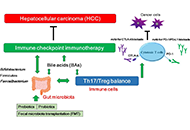
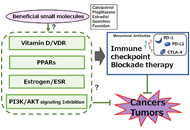
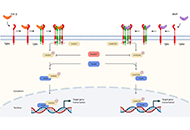
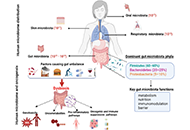
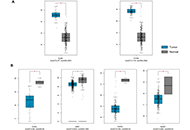
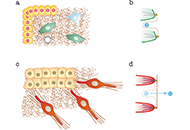
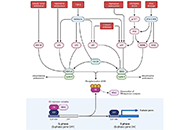
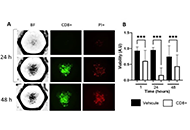
Immunotherapy is poised to become an increasingly used strategy for the treatment of cancer. Targeting immune checkpoints, such as programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), has led to unprecedented clinical outcomes in several cancer patients. However, despite the favorable outcome for immune checkpoint blockade (ICB)-responding patients, the response rate remains low. This is in part due to the influence of the tumor microenvironment (TME) in protecting malignant cells from the antitumor immune response and in facilitating immune escape. A paradoxical coexistence of tumor antigen-specific CD8 T lymphocytes and tumor growth arises from multiple negative immunoregulatory pathways that impede T cell-mediated tumor destruction. Indeed, cancer cells develop multiple immunosuppressive mechanisms to resist to antitumor immunity. The role of the tumor ecosystem during the initiation and progression of carcinogenesis is presently considered to be of critical importance. Therefore, targeting the TME-associated pathways may offer new options in the design of innovative cancer immunotherapy approaches. In this respect, immunotherapies could be more effective by combination of ICB and/or therapeutic cancer vaccines with agents that modulate the TME in order to overcome tumor tolerance and immune resistance, which are two major key issues that need more attention. This special issue aims to provide a comprehensive review covering some recent developments in the regulation of antitumor immunity and cancer immunotherapy, and the impact of the TME on tumor resistance and immune suppression. It also offers some perspectives on how exploring cancer cells that evade the immune surveillance may be helpful to develop more efficient strategies to target immune-escaped tumors and to design more adapted and integrative immunotherapies. The concept of reinvigorating the antitumor immune response by targeting the tumor ecosystem will certainly improve current cancer care.
Keywords: Onco-immunology; cancer immunotherapy; tumor microenvironment; T lymphocytes; MDSC; CAF; tumor resistance
Published Articles